Mar 08 2022
Solid State Li-ion Batteries
 OK, I’m a sucker for juicy battery news, even though I know these advances are all incremental and not the dramatic “breakthrough” they are often presented to be. But cumulatively these incremental advances are significantly increasing the energy per mass and energy per volume of high-end batteries. This is a critical technology for our low-carbon future, and so these advances are worth tracking.
OK, I’m a sucker for juicy battery news, even though I know these advances are all incremental and not the dramatic “breakthrough” they are often presented to be. But cumulatively these incremental advances are significantly increasing the energy per mass and energy per volume of high-end batteries. This is a critical technology for our low-carbon future, and so these advances are worth tracking.
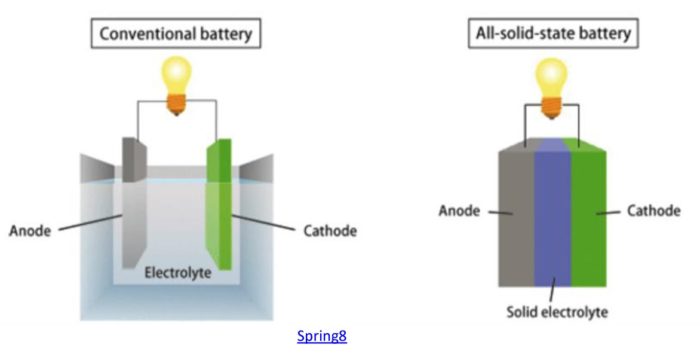
As I always point out when I write about battery technology, an ideal battery needs to have many features simultaneously: good specific energy, energy density, lifespan in terms of charge-discharge cycles, rapid recharge rates, sufficient power, use of cheap, abundant, and non-toxic materials, a good temperature range of operation, overall cost-effectiveness, scalable manufacturing, and stability (it’s nice if they don’t spontaneously burst into flames). Li-ion batteries are the current state-of-the-art because they are reasonable to good on all these parameters, but there is a lot of room for improvement. One of the many lines of research looking into alternate battery design is solid state Li-ion batteries. These use a solid rather than liquid electrolyte for conducting charge between the electrodes.
A solid state design could have twice the specific energy (energy per mass) and twice the energy density (energy per volume) as current Li-ion batteries. This is because the solid electrolyte requires much thinner separators between the electrodes, and also because the lithium-graphite electrode can be replaced with a lighter pure lithium electrode. Solid state batteries also will likely have a longer lifespan and are more stable and therefore safer. Imagine essentially doubling the capacity of a lithium battery, for your cellphone, laptop, or electric vehicle. You could increase the range of an EV by 50% while still decreasing the battery weight by 25% (which would actually increase the range a bit more). I own an EV with a range of 350 miles, and going to a range of 500 miles would definitely reduce range-anxiety on long trips. That might be the sweet spot for EVs.
But we have not achieved a workable solid state Li-ion battery yet. There are essentially two major obstacles that need to be overcome. One is the lower conductivity of the solid electrolyte compared to the liquid electrolyte. But this problem has essentially been solved by finding materials with a sufficiently high conductivity. So only the second hurdle remains – which is instability at the interface between the solid electrolyte and the electrodes. This instability causes dendrite growth which rapidly degrades the lifespan of the battery. It’s basically a deal-killer until it can be solved. There is already one partial solution, to coat the electrodes with a material that reduces this dendrite growth. But the coating introduces an expensive process into the manufacturing of the batteries, and so won’t work for mass production.
A new study, however, introduces a new process to address the instability issue, that requires only negligible extra expense and appears to work as well or better than the coatings. The solution stems from identifying the problem, which is impurities introduces in the sintering process. Solid state batteries use ceramics as structural material for the electrodes and the electrolyte. These ceramics are sintered together during manufacturing, which is a process of heating up the ceramics so that they become soft and sticky without fully melting them. The soft ceramics are then stuck together to make a good contact with good conduction. However, at the temperatures required for sintering CO2 from the air can get incorporated into the material, forming compounds that impede conduction. This results in a bottleneck for the conduction of electricity in the battery, which is greatest at the interface between the solid electrolyte and the cathode.
The current study looks at Li7La3Zr2O12 (LLZO) solid electrolyte with a thin film LiNi0.6Mn0.2Co0.2O2 cathode. The LLZO electrolyte is currently one of the best candidates for a solid state Li-ion battery because it has high conduction. The solution to the problem of high temperatures introducing CO2 impurities is to simply remove CO2 from the air in the sintering process. This is done by performing the sintering in a pure oxygen atmosphere. The authors write:
“Sintering in O2 is ideal, yielding excellent chemical stability and low interfacial resistance. Secondary phases also do not form in N2, but oxygen loss occurs at elevated temperatures. The interfacial resistance obtained upon sintering in pure O2 is comparable to the lowest values at LLZO interfaces with protective coatings, but here without the need for interface coatings.”
They compared pure O2, pure nitrogen, humidified air, and normal atmosphere which contains a small amount of CO2. The pure oxygen atmosphere had the best results. Researcher still have to fully test batteries made with this process, for longevity and other properties. But it does seem like we are one step closer to solid state Li-ion batteries, which could be a big jump in battery technology.






