 By now many people have heard of graphene, an allotrope of carbon that is “2-Dimensional”, meaning that it is a sheet of carbon one atom thick. The carbon atoms are arranged like in a chicken wire. Graphene is considered a modern “wonder material” because it is flexible yet strong and has superior temperature and electrical conducting properties. You can also “dope” the graphene with many other elements to create novel properties. We are really just at the beginning of exploring the potential of this material, but already it us being used as an additive to make materials like plastic stronger and more conductive. A limiting factor is the ability to manufacture graphene in bulk and at high quality (with few errors in the arrangement of carbon atoms), but research is extensive because of the incredible potential of the material.
By now many people have heard of graphene, an allotrope of carbon that is “2-Dimensional”, meaning that it is a sheet of carbon one atom thick. The carbon atoms are arranged like in a chicken wire. Graphene is considered a modern “wonder material” because it is flexible yet strong and has superior temperature and electrical conducting properties. You can also “dope” the graphene with many other elements to create novel properties. We are really just at the beginning of exploring the potential of this material, but already it us being used as an additive to make materials like plastic stronger and more conductive. A limiting factor is the ability to manufacture graphene in bulk and at high quality (with few errors in the arrangement of carbon atoms), but research is extensive because of the incredible potential of the material.
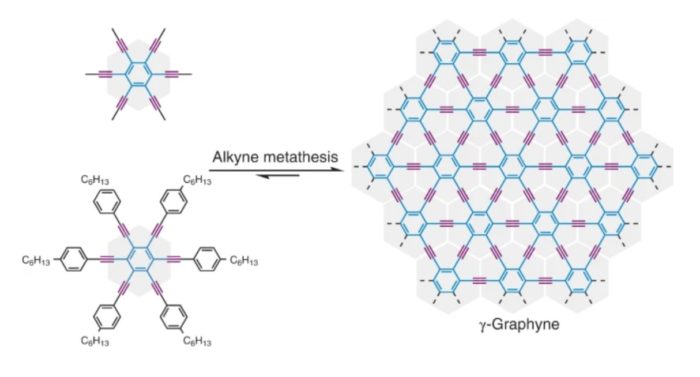
Before graphene has really hit its stride, scientists have now made for the first time a related carbon allotrope called graphyne. This is also a 2-Dimensional material with similar amazing properties like graphene, but may be even better. Previous scientists have only been able to make nanometer scale amounts of graphyne, but a recent study report the production of graphyne in bulk. Graphyne has a different arrangement of carbon atoms than graphene. It is more complex and combines different types of carbon binding. That was the trick, to combine different types of carbon in the same material.
Carbon is an extremely useful element (and the basis of organic life) because of its unique properties. It has four available electrons with which it can form bonds with other elements, in either single, double, or triple bonds, which creates a lot of potential configurations. Carbon bonds are either sp3, sp2, or sp, depending on which electrons orbits are being used to share electrons and form a bond. You can read the details here if you are interested, but it has to do with the specific type of bonds using the different orbitals. Graphite uses only sp2 bonds, while diamond uses sp3 bonds (both are allotropes of carbon). Graphene is also sp2.
Graphyne, on the other hand, combines sp and sp2 carbon atoms which can give it useful properties:
Unlike graphenes, which consist solely of sp2-hybridized carbons, graphynes contain sp-hybridized carbons periodically integrated into an sp2-hybridized carbon framework. It was predicted that graphyne would exhibit intriguing and unique electron-conducting, mechanical and optical properties. Specifically, the electron conduction in graphynes would be exceptionally fast, as it is in graphene. Yet, the electron conduction in some graphynes could be controlled in a defined direction, unlike the multidirectional conduction in graphene, because the triple bonds can create distortion in Dirac cones.
Essentially graphyne has all the cool properties of graphene – it’s light, strong, and highly conductive to electricity and heat – but has the additional property of having controllable unidirectional conduction. Graphene, on the other hand, conducts in all directions. Controlling the direction of the conduction of electricity is critical to many electronic applications.
We are still in the “potential” stage here. Graphyne is getting a lot of attention because chemists predicted for years that it would be an incredibly useful material for electronics, but no one could manufacture it. Now a group has used a new technique to make it in bulk, which has reignited interest in the material. Further, now researchers can get their hands on the material and begin to explore its actual properties (not just theoretically predicted properties).
In the meantime, further research is needed to refine the production process, to make it quicker and cheaper for hopefully industrial scale production. This is often a deal-killer for new technologies. If you cannot produce it at an industrial scale it will remain a laboratory curiosity, or at best a high-end niche application. We may see it is probes NASA sends to the outer solar system, but not in your home or car.
Both graphene and graphyne still command incredible attention and investment because of their potential to revolutionize several industries. They could be used to make batteries with far greater energy density and specific energy than current lithium-ion batteries. They could be used in micro-optics, in superfast computers, and also wearable technology (because the materials are thin and strong). As it typically the case hype tends to get ahead of reality by 10-20 years. It takes time to work out the kinks and figure out how to make a technology actually work. It’s also possible we may never figure out a way to mass produce either material in sufficient quantities and qualities to realize the most promising applications, but given how the field is progressing we can be optimistic.
Graphyne now adds a new dimension to the promise of these new carbon 2-dimensional materials.
 If you haven’t seen the new series, Prehistoric Planet, hosted by David Attenborough, you should see it. The visuals are stunning, the science is updated, and it provides a compelling look into the world of dinosaurs and other animals contemporary to the dinosaurs. While watching an episode last night, depicting a velociraptor leaping around energetically, my wife asked, “Were dinosaurs cold-blooded?” The classic concept of dinosaurs is of large lumbering and slow animals, cold-blooded (ectothermic) like other reptiles. However, scientists have long suspected that some if not all dinosaurs may have been warm-blooded (endothermic) – hence the updated vision of dinosaurs as energetic animals. (The picture is of a baby T-Rex.)
If you haven’t seen the new series, Prehistoric Planet, hosted by David Attenborough, you should see it. The visuals are stunning, the science is updated, and it provides a compelling look into the world of dinosaurs and other animals contemporary to the dinosaurs. While watching an episode last night, depicting a velociraptor leaping around energetically, my wife asked, “Were dinosaurs cold-blooded?” The classic concept of dinosaurs is of large lumbering and slow animals, cold-blooded (ectothermic) like other reptiles. However, scientists have long suspected that some if not all dinosaurs may have been warm-blooded (endothermic) – hence the updated vision of dinosaurs as energetic animals. (The picture is of a baby T-Rex.)
 I guess some good came out of Brexit. The EU essentially has banned GMO (genetically modified organisms) products, which I believe is
I guess some good came out of Brexit. The EU essentially has banned GMO (genetically modified organisms) products, which I believe is  Memory research, both at the psychological and neurological level, is fascinating, partly because memories are so essential to who we are. We often don’t perceive the underlying mechanisms by which memories are formed, stored, and recalled, but they dramatically affect our mental life. Further, being aware of how our memories work is a critical part of neuropsychological humility – human memory is not perfect, it’s not like a tape recorder, it is a dynamic and flawed process. For example, different aspects of a memory and different memories are linked together, but these connections can be jumbled. We may fuse the detail of one memory to another, alter details completely, and even remember things that happened to someone else as if they happened to us.
Memory research, both at the psychological and neurological level, is fascinating, partly because memories are so essential to who we are. We often don’t perceive the underlying mechanisms by which memories are formed, stored, and recalled, but they dramatically affect our mental life. Further, being aware of how our memories work is a critical part of neuropsychological humility – human memory is not perfect, it’s not like a tape recorder, it is a dynamic and flawed process. For example, different aspects of a memory and different memories are linked together, but these connections can be jumbled. We may fuse the detail of one memory to another, alter details completely, and even remember things that happened to someone else as if they happened to us. By now many people have heard of graphene, an allotrope of carbon that is “2-Dimensional”, meaning that it is a sheet of carbon one atom thick. The carbon atoms are arranged like in a chicken wire. Graphene is considered a modern “wonder material” because it is flexible yet strong and has superior temperature and electrical conducting properties. You can also “dope” the graphene with many other elements to create novel properties. We are really just at the beginning of exploring
By now many people have heard of graphene, an allotrope of carbon that is “2-Dimensional”, meaning that it is a sheet of carbon one atom thick. The carbon atoms are arranged like in a chicken wire. Graphene is considered a modern “wonder material” because it is flexible yet strong and has superior temperature and electrical conducting properties. You can also “dope” the graphene with many other elements to create novel properties. We are really just at the beginning of exploring  Demand for electric vehicles (EVs) is increasing, but still there is lingering hesitancy to make the switch to EVs. Sales of EVs have been
Demand for electric vehicles (EVs) is increasing, but still there is lingering hesitancy to make the switch to EVs. Sales of EVs have been  Having a working understanding of the biases and heuristics that our brains use to make sense of the world is critical to neuropsychological humility and metacognition. They also help use make better sense of the world, and therefore make better decisions. Here’s a fun example. Let’s say you increase your driving speed from 40 mph to 60 mph over a 100 mile journey. How much would you need to increase your speed from a starting point of 80 mph in order to save the same amount of time on the journey? Is it 100 mph or 120 mph?
Having a working understanding of the biases and heuristics that our brains use to make sense of the world is critical to neuropsychological humility and metacognition. They also help use make better sense of the world, and therefore make better decisions. Here’s a fun example. Let’s say you increase your driving speed from 40 mph to 60 mph over a 100 mile journey. How much would you need to increase your speed from a starting point of 80 mph in order to save the same amount of time on the journey? Is it 100 mph or 120 mph? What if there were a change we could make in our society that would save, in the US alone, more than 50,000 lives per year and avoid more than $600 billion every year in health care costs and lost productivity? How much should we invest each year to make the necessary changes? Even if we invested $3 trillion over the next 10 years, that would only be half as much as we would save over the same length of time (in addition to preventing have a million premature deaths). Would you say that was worth it? It sounds like a good deal, but of course we have to delve into the details.
What if there were a change we could make in our society that would save, in the US alone, more than 50,000 lives per year and avoid more than $600 billion every year in health care costs and lost productivity? How much should we invest each year to make the necessary changes? Even if we invested $3 trillion over the next 10 years, that would only be half as much as we would save over the same length of time (in addition to preventing have a million premature deaths). Would you say that was worth it? It sounds like a good deal, but of course we have to delve into the details. After years of requesting tiny samples of lunar soil,
After years of requesting tiny samples of lunar soil, 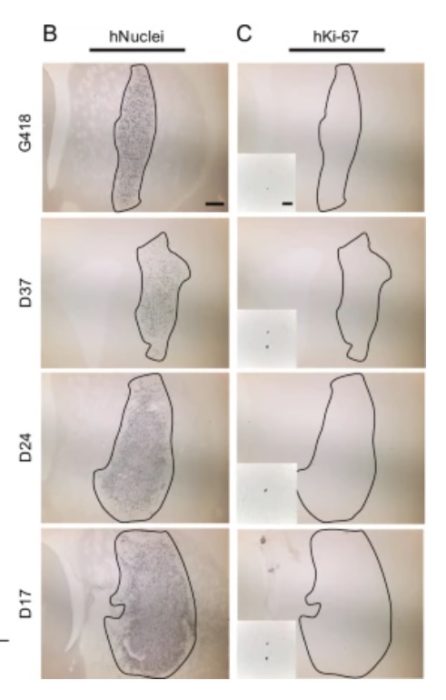 The healing potential of stem cells came to the public consciousness in 2001, when president George Bush
The healing potential of stem cells came to the public consciousness in 2001, when president George Bush Human behavior is complex and can be very difficult to predict. This is one of the challenges of safe driving – what to do when right of way, for example, is ambiguous, or there are multiple players all interacting with each other? When people learn to drive they first master the rules, and learn their driving skills in controlled situations. As they progress they gain confidence driving in more and more complicated environments. Even still there are about
Human behavior is complex and can be very difficult to predict. This is one of the challenges of safe driving – what to do when right of way, for example, is ambiguous, or there are multiple players all interacting with each other? When people learn to drive they first master the rules, and learn their driving skills in controlled situations. As they progress they gain confidence driving in more and more complicated environments. Even still there are about 




