Feb 01 2021
Protein Switches and COVID Testing
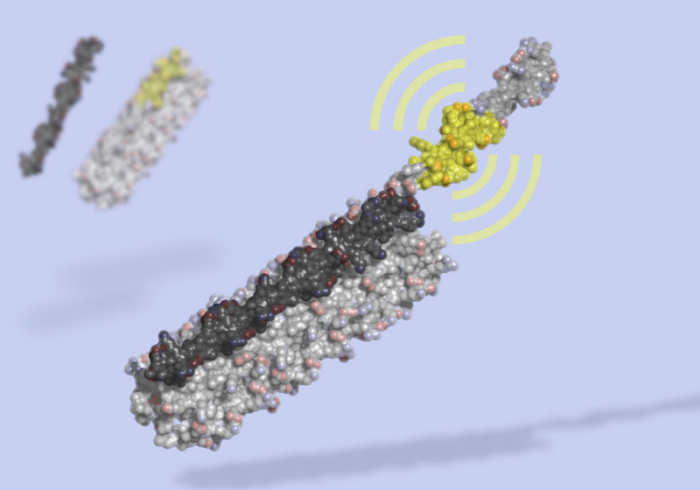 Researchers report in Nature the development of a new technique for designing protein switches that can be used as biosensors. The recent development of the technology to design specific protein switches is an underreported story, in my opinion, and represents a technology with incredible possibilities. Reporting on the recent study emphasizes one possible application – development of a new rapid test for SARS-CoV-2. It is understandable why this would garner the most interest – but the underlying technology is perhaps a bigger science news story.
Researchers report in Nature the development of a new technique for designing protein switches that can be used as biosensors. The recent development of the technology to design specific protein switches is an underreported story, in my opinion, and represents a technology with incredible possibilities. Reporting on the recent study emphasizes one possible application – development of a new rapid test for SARS-CoV-2. It is understandable why this would garner the most interest – but the underlying technology is perhaps a bigger science news story.
A protein switch is simply a protein that can change its 3-dimensional configuration in response to binding with something, such as another protein or a hormone or some biological signal. When a protein changes its configuration, it changes its function. This can turn a function of the protein on or off, open or close a pore or channel, or alter its activity. Protein switches are a basic component of biological function as they allow for the sensing of internal biological states and reaction to those states by altered cellular function.
It was only in 2019, less than two years ago, that scientists reported the design and creation of the first completely artificial protein switch. Again, this story did not make a huge splash, but looking back this may have been as momentous as the development of CRISPR as a tool for genetic engineering. It’s hard to tell how much of a long term impact it will have – but just as CRISPR (and related tools of genetic engineering) gives us unprecedented control over a fundamental aspect of biology (genetics), protein switches also potentially give us a similar level of control, arguably more direct.
Also similar to CRISPR, the technology of designing protein switches is advancing quickly. The new Nature study reports on a technique for module and tunable protein switches – ones that can quickly be tuned with high sensitivity and specificity to a specific protein. The switch changes configuration upon binding from a closed dark state to an open state that glows with bioluminescence. The application here is obvious – if the protein in question is present, the switch will glow. Proteins, of course, include antibodies, or parts of virses.
The researchers were already able to apply this modular system to various proteins:
We demonstrate the modularity of this platform by creating biosensors that, with little optimization, sensitively detect the anti-apoptosis protein Bcl-2, the IgG1 Fc domain, the Her2 receptor, and Botulinum neurotoxin B, as well as biosensors for cardiac Troponin I and an anti-Hepatitis B virus (HBV) antibody that achieve the sub-nanomolar sensitivity necessary to detect clinically relevant concentrations of these molecules
Without getting into all the details, the important bits here are that these are all distinct and medically important proteins, and the sensitivity of the assay is at extremely low concentrations that are biologically relevant. Perhaps sensing what would most likely give them press attention, the researchers then moved onto COVID:
Given the current need for diagnostic tools for tracking COVID-19, we used the approach to design sensors of antibodies against SARS-CoV-2 protein epitopes and of the receptor-binding domain (RBD) of the SARS-CoV-2 Spike protein. The latter, which incorporates a de novo designed RBD binder, has a limit of detection of 15 pM and a signal over background of over 50-fold.
That’s is extremely good sensitivity, and a great signal to noise ratio. But the researchers emphasize that this one application is not the real story – which rather is the modular design of this technique. With it they should be able to quickly and cheaply develop novel protein switches that could be used for a huge variety of medical applications. Time will tell, and it is always easier to evaluate the impact of new technologies in retrospect. When the polymerase chain reaction (PCR) first came out, it was exciting as the potential was clear. Now we can look back and see that it totally lived up to the initial hype. PCR can be used to amplify tiny amounts of genetic material to produce a signal that can be detected, and is now widely used in medicine as well as forensics. Sometimes initial hype is justified.
Another example of this is monoclonal antibodies, the ability to make a large amount of one specific type (clone) of antibody. Although I first heard about them 30 years ago, we are now seeing a slew of monoclonal antibody therapeutics hitting the market.
I hope that, if anything, modular designer protein switches is being underhyped. This may prove to be an extremely important new class of not only medical diagnostics but therapeutics. While a good rule of thumb is that new basic discoveries take 2-3 decades to really hit the clinic, this pace may be increasing significantly. We are already into clinical trials with CRISPR therapeutics. We may see rapid and sensitive lab tests based on modular protein switches for COVID-19. Part of the reason for this accelerating of development is that these new techniques (such as CRISPR and protein switches) are designed to be quick and easy.
One downside, however, is that biological therapeutics are still really expensive, but not necessarily more expensive than some new chemical drugs. The new monoclonal antibody treatments for migraines, for example, can cost about $7,000 a year. Biologics are just expensive. Given the rising cost of healthcare, which is mostly driven by new technology, we have to keep an eye on this factor as well.






