Oct 10 2022
Nitrous Oxide as a Biosignature
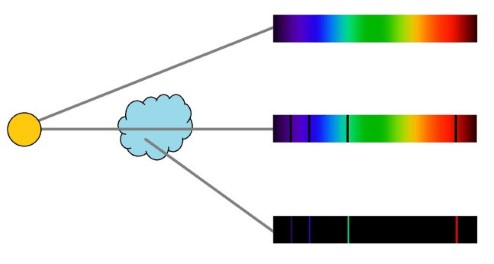 I still find it amazing that we can look at an astronomical object light years away and determine its chemical composition in detail. This is referred to as spectral analysis – looking at either absorption or emission lines in the wavelengths of light. Isaac Newton was the first to demonstrate that white light from the sun is actually composed of the full spectrum of visible light, which can be separated by passing through a prism which bends light of different colors to different degrees, causing it to spread out into the familiar rainbow pattern. However, Newton was not the first to discover this effect, but prior to his experiments it was believed the prisms colored the light. Newton demonstrated that the colors were already there.
I still find it amazing that we can look at an astronomical object light years away and determine its chemical composition in detail. This is referred to as spectral analysis – looking at either absorption or emission lines in the wavelengths of light. Isaac Newton was the first to demonstrate that white light from the sun is actually composed of the full spectrum of visible light, which can be separated by passing through a prism which bends light of different colors to different degrees, causing it to spread out into the familiar rainbow pattern. However, Newton was not the first to discover this effect, but prior to his experiments it was believed the prisms colored the light. Newton demonstrated that the colors were already there.
In 1802, William Hyde Wollaston improved on the prism design to produce more detailed spectra. He discovered black bands of missing wavelengths in the light. These are absorption lines – chemicals will absorb specific wavelengths of light depending on their chemical structure (corresponding to the orbits of electrons which absorb the energy of light to jump to a higher energy orbit), with the pattern of absorption lines being a signature of the specific chemical. There are also emission lines in which specific wavelengths of light are created depending on the source of the light. Therefore we can tell the chemical composition of a star by looking at its emission lines, and we can also tell its temperature as different elements and chemical have peak emissions at different temperatures. When starlight passes through a gas cloud and material in the cloud will absorb specific frequencies of light, telling us what it is made of.
Therefore, if an exoplanet with an atmosphere is discovered through the transit method (it causes the light of its star to dim when it blocks a small amount of it as it passes in front), then we may be able to detect some of the light from that star as it passes through the atmosphere of the planet. This requires high resolution imaging, but within our current capabilities for some exoplanets (and now extended with the James Webb Space Telescope). With spectral analysis we get information about the composition and temperature of the exoplanet’s atmosphere. Astronomers are most interested in using this method to look for signs of life (so-called biosignatures). What would a sign of life in a planet’s atmosphere be?
The basic concept of a biosignature is something we can detect that likely originated from the activity of living organisms. Living things are essentially chemical factories, and they produce byproducts that can accumulate in the atmosphere of a planet, given enough time. The most consequential such byproduct on Earth is oxygen, which is created by photosynthesizing life, at first bacteria and eventually plants. Plants and cyanobacteria breath CO2 which they combine with water to create sugars for food, creating oxygen as a waste byproduct that eventually builds up in the atmosphere. Another biosignature chemical found on Earth in methane, from animal flatulence.
Oxygen and methane are biosignatures also because they are highly reactive compounds, which means they would not be found in the atmosphere unless they were continuously being replenished. In Mars, for example, any oxygen in its atmosphere has reacted with iron and other metals in the crust, turning the surface red but also eliminating oxygen from the atmosphere. The question is – are there any non-living (abiotic) sources of oxygen or methane that could produce a continuous supply? These geological sources could create a false biosignature, and astronomers always have to rule them out before declaring they have found a true biosignature.
A recent paper argues that we need to add another chemical to the list of possible atmospheric biosignatures – nitrous oxide (N2O). Some living organisms produce nitrates as a byproduct of their metabolism. Other living things can further metabolize nitrates to create N2O. On Earth there is only a very small amount of N2O, likely too little to be detected in exoplanets. What the paper explores, however, is modeling different amounts of N2O creation and buildup at different historical times on Earth and under different atmospheric conditions (mainly different levels of oxygen). They find that under certain conditions N2O flux could be much higher, leading to detectable amounts of N2O in an exoplanet’s atmosphere. That would mean that the exoplanet more closely resembles Earth at earlier times in its evolution. The paper also explores abiotic sources of N2O. This includes lightening strikes – however, this method would only produce small amounts of N2O. It would also produce nitrogen dioxide, and so the presence of that chemical would suggest an abiotic source.
The overall concept is that we should not limit ourselves to biosignatures detectable on present-day Earth. We need to consider other possible configurations of life, and at different potential times in the evolution of planets. In the past, N2O may have been a biosignature for aliens observing Earth. Essentially any potential biotic process producing a chemical that can build up to detectable levels in the atmosphere is a potential biosignature. These are more useful if they are short-lived, meaning they have to be actively replenished, and if they have no significant abiotic source (or potential abiotic sources have other signatures which give themselves away).
It seems reasonable to add N2O to the list, and there may be others as well.






