Jun 04 2015
Batteriser – Cool Tech or Scam?
 A company is claiming that they have created a small sleeve costing only $2.50 that will fit over standard alkaline batteries, and boost their life by up to 800%. My initial reaction to this claim is that it is almost certainly total BS. But I am always willing to give a new claim a fair shake and dive into the details.
A company is claiming that they have created a small sleeve costing only $2.50 that will fit over standard alkaline batteries, and boost their life by up to 800%. My initial reaction to this claim is that it is almost certainly total BS. But I am always willing to give a new claim a fair shake and dive into the details.
The reasons for my initial skepticism are many. Energy scams are common. They often promise incredible effects from implausible devices. Usually, by asking one simple question, you can destroy the claims utterly – from where is the extra energy coming? The laws of thermodynamics are called “laws” for a reason, and reality is a harsh mistress.
Bob Roohparvar, owner of Batteroo which is developing the Batteriser, has an answer to this question. He claims that typical alkaline batteries use about 20% of their available energy. The reason for this high inefficiency is that electronic devices are finicky; they need a steady voltage of 1.5. When an alkaline battery drops below 80% capacity the voltage also drops to 1.4 or 1.3. At that point the device will read the battery as dead, leaving 80% of its juice unused.
The Batteriser, Roohparvar claims, is a miniaturized device that boosts the voltage back up to 1.5, and maintains it there until every last drop of energy is extracted from the battery. OK, I thought, at least he has an answer to the most basic question about such claims. But that is about all he has. Even a slightly deeper dive into battery technology and his claims reveals them to be untenable.
Before I deliver the coup de grace, my skepticism about the Batteriser grew the more I read Roohparvar’s claims. First, simple math told me that if batteries normally have a 20% efficiency and he is extracting 100%, that is a 5x improvement, not an 8x improvement. So how does he get to 8x?
Let’s say you buy a new battery. You use it for a month and its voltage drops to 1.4. It’s now ostensibly dead at 1.4 volts, but if you slip on a Batteriser, its output increases to 1.5 volts for another month. That’s already a 2x increase in battery life. Eventually the battery’s natural, unboosted output drops to 1.3 volts—but Batteriser keeps it at 1.5 volts for another month. Now you’ve realized a 3x increase in battery life. And so on, and so on. Roohparvar says Batteriser can continue to deliver a 1.5 volt charge in batteries that have discharged down to 0.6 volts. There are more than eight 0.1 volt steps between 0.6 and 1.5 volts, so, in grossly simplified terms, the Batteriser can extend operational battery life somewhere around a factor of eight.
I call BS on that. His fancy math does not get you around the fact that 100% energy is 5 times 20% energy. He is playing a little shell game. It seems likely, assuming his device even works, that as you extract higher voltage from the battery it will drain faster and faster. In fact, new inefficiencies might be introduced. The way to boost voltage will be to draw more current from the battery, which will heat the battery more, and might make it less efficient (in addition to being a potential hazard). In any case, the 800% claim is almost certainly nonsense, even by Roohparvar’s own logic.
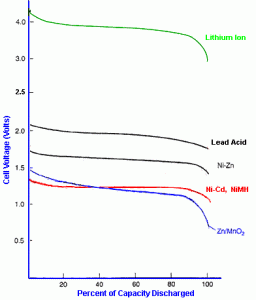 But can, then, the Batteriser extract 500% more life out of those AAs? I don’t think so, and here’s why. While it is true that the voltage of an alkaline battery (and all batteries) drops off as capacity drops, the big drop off doesn’t occur until around 82% of the capacity has been drained, and therefore only about 18% remains. Take a look at the graph – Zn/MNO2 is alkaline batteries, the blue line at the bottom. You can see that over the first 80% of the battery’s life the voltage slowly decreases from 1.5 to about 1.2 volts. Just above 80% it then drops off the cliff.
But can, then, the Batteriser extract 500% more life out of those AAs? I don’t think so, and here’s why. While it is true that the voltage of an alkaline battery (and all batteries) drops off as capacity drops, the big drop off doesn’t occur until around 82% of the capacity has been drained, and therefore only about 18% remains. Take a look at the graph – Zn/MNO2 is alkaline batteries, the blue line at the bottom. You can see that over the first 80% of the battery’s life the voltage slowly decreases from 1.5 to about 1.2 volts. Just above 80% it then drops off the cliff.
The question then is – what is the cutoff voltage for most devices? You can look this up yourself. I found a good reference at Popular Mechanics, although it was from 1994. The types of devices that still use alkaline batteries, however, are roughly the same as then. Newer electronic devices, like cell phones and iPads, run on lithium ion batteries. Here is their table:
autofocus camera with flash: 1.3v
halogen flashlight: 1.3v
regular flashlight: 1.2v
headphone/CD player: 1.2v
portable LCD TV: 1.2v
pencil sharpener: 1.2v
AM/FM radio: 1.0v
electric shaver: 1.0v
quartz clock: 1.0v
remote control: 0.8v
You can see that the devices cutoff starting at 1.3v and as low as 0.8v. Except for the high-end devices, most of the devices cutoff at 1.2v or lower, meaning they will extract 80% or more of the stored energy from an alkaline battery. I looked up the cutoff voltage for a couple of more modern devices for comparison. Microsoft cordless optical mice have a cutoff voltage of 0.6v, and Logitech mice of 0.8v. These are therefore very efficient devices.
Also, keep in mind that many devices have battery packs with multiple batteries. The voltage of each battery is added – so if you have a battery pack with four AA alkaline batteries they will generate 6.0 volts when fresh. Let’s say you are using a device that requires 4.0 volts, that means the batteries will last until they are drained down to 1.0v, which is again north of 80% efficiency.
This means it is largely up to the device manufacturer to design the device to operate efficiently with available batteries – designing the battery pack and the cutoff voltage to meet the needs of the device and optimize the efficiency of the batteries. There certainly can be devices out there that are poorly designed, with high cutoff voltage and/or too small a battery pack that are highly inefficient. It does not seem as if this is typical, however.
All of this means that the Batteriser, if it works optimally and does squeeze every last drop out of alkaline batteries (and does so safely) is probably going to increase typical battery life by about 20%, not 800%. A 20% increase in battery life is not bad, but it probably won’t lead to the successful Indigogo campaign that Roohparvar is counting on. It also may not be cost effective or worth the bother of using the Batteriser (depending on the device). The product may have a niche application for specific devices that have a necessarily high cutoff voltage, but it is not going to revolutionize battery tech or save the world, as the hype promises.
Once again the smell test for overhyped energy tech claims seems to be accurate. The claims seemed too good to be true because they were.
Of course, I am wiling to be proven wrong. I would be happy to test the Batteriser for myself in various devices, and if any of my arguments are not sound please point out my error.






