Mar 25 2022
Using CRISPR to Turn Genes On
 Last year I wrote about CRISPR On-Off – this is a system for using the genetic modification tool, CRISPR, in order to turn the expression of a gene off and then back on again, without altering the gene itself. Now researchers have published a similar application of CRISPR, using a different mechanism to turn on the expression of silenced genes. Their technique shows the power of CRISPR as a modifiable platform. The research also used AI to design the new system, again showing how artificial intelligence is being used to dramatically speed up the pace of research.
Last year I wrote about CRISPR On-Off – this is a system for using the genetic modification tool, CRISPR, in order to turn the expression of a gene off and then back on again, without altering the gene itself. Now researchers have published a similar application of CRISPR, using a different mechanism to turn on the expression of silenced genes. Their technique shows the power of CRISPR as a modifiable platform. The research also used AI to design the new system, again showing how artificial intelligence is being used to dramatically speed up the pace of research.
The new technique also uses CRISPR (Clustered regularly interspaced short palindromic repeats), which was derived from bacteria that use it as part of their immunity against viruses. CRISPR is like a carrier, which can be attached to a specific stretch of DNA. It will then find that stretch of DNA within a genome and target it. CRISPR can also be attached to a variety of proteins, most famously CAS9, which can then perform some function when it gets to its target. CAS9 is a DNA splicer, so a CRISPR-CAS9 system can target a desired stretch of DNA and splice it. This can be used to disrupt a gene, or it can be used to create a location for the insertion of a new gene or gene modification, which requires a separate process involving the DNA repair mechanism.
The CRISPR system dramatically reduced the cost and time necessary to make alterations to a genome. The technology is also rapidly progressing, because research using CRISPR is now available to many more labs and researchers. There are other payloads other than CAS9 that can be used, for example. Researchers are also learning how to tweak the speed vs accuracy of CRISPR.
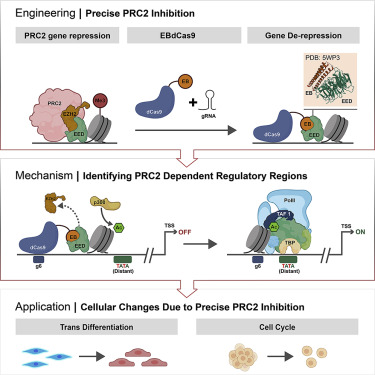
The current researchers wanted to develop another way to increase the express of genes that have been effectively silenced. This is a great way to study what those genes do, especially if they can be turned on individually. As cells develop they selectively turn on and off genes to determine their function. Every nucleated cell in the body has the full genome, but there are liver genes that make some cells develop into liver cells, and lung genes that make other cells turn into lung cells. One system to suppress the expression of a gene is histone 3 lysine 27 methylation (H3K27me3) marks propagated by the polycomb repressive complex 2 (PRC2). Histones are proteins that wrap up the long DNA molecule to keep in manageable within cells. PRC2 is a protein that essentially block a gene from being expressed. If you could block PRC2, therefore, you could turn a repressed gene on. So that is what the researchers did.
First they started with CRISPR-CAS9. They then modified the CAS9 so that it no longer functioned, turning it into dCAS9 (“d” is for “dead”). They then used AI to design a protein that would block the function of PRC2, and attached that protein to the dCAS9. They were then able to increase the expression of selective genes one at a time by targeting them with the CRISPR component. Here’s what they found:
In total, we have targeted 26 specific sites in promoter regions upstream of four different genes and observed significant PRC2-dependent transcriptional de-repression in 8 loci. NCdCas9, which differs from EB by two amino acids required for EED binding, does not result in transcriptional changes. This suggests that EBdCas9 functions by locally inhibiting PRC2 activity.
The “EB” was the protein designed to block the function of PRC2, which worked when applied to 8 of the studies locations on the target DNA to increase gene expression. As a control they used a different protein, NC, which did not alter gene activity (transcription from the gene into a protein). This supports the conclusion that it was the blocking of the PRC2 that was important. This study was more about testing out how the whole system works, rather than studying the efficacy of specific applications. In short they showed that their EBdCAS9 system works.
What is powerful about this system, and CRISPR in general, is that it is not one application but a platform. We have CRISPR, which can target a specific desired location within a DNA strand in a cell. Connected to that is dCAS9, which essentially functions like a connector segment attaching the CRISPR to another protein. Proteins can then be designed for specific functions, as in this case where EB was designed to block PRC2. This then functions as a modular system with interchangeable parts for different applications. Further still, we can use AI algorithms to design specific proteins for specific functions. So it’s a designer modular system. Pretty cool.
This again shows the incredible power of modern genetics. We are increasingly taking control of the fundamental code of life, able to alter it at will, and we’re just getting started.






