Apr 13 2021
A CRISPR Genetic On-Off Switch
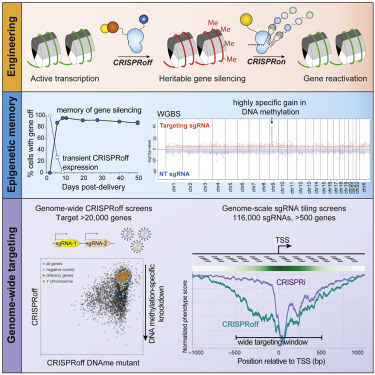 Our knowledge of genetics and the tools to engineer or modify genetics continues to rapidly progress. The most celebrated recent advance was CRISPR (clustered regularly interspaced short palindromic repeats), a bacteria-derived system that can easily target any sequence of DNA using a guide RNA. CRISPR is like the targeting system and it can be paired with various payloads, most commonly Cas9, which is an enzyme that will cut both strands of DNA at the desired location. CRISPR was actually discovered in 1993, but the CRISPR-Cas9 system was first used for gene editing in 2013, an advance that won the Nobel prize in chemistry in 2020.
Our knowledge of genetics and the tools to engineer or modify genetics continues to rapidly progress. The most celebrated recent advance was CRISPR (clustered regularly interspaced short palindromic repeats), a bacteria-derived system that can easily target any sequence of DNA using a guide RNA. CRISPR is like the targeting system and it can be paired with various payloads, most commonly Cas9, which is an enzyme that will cut both strands of DNA at the desired location. CRISPR was actually discovered in 1993, but the CRISPR-Cas9 system was first used for gene editing in 2013, an advance that won the Nobel prize in chemistry in 2020.
We are still, however, on the steep part of the learning curve with this powerful technology, and now researchers have published perhaps the greatest advance since 2013 – a way to use CRISPR as an on-off switch for genes. At the very least this will revolutionize genetic research. But it also has incredibly therapeutic potential, although other hurdles remain for applications in living organisms.
Using CRISPR-Cas9 for gene editing basically comes in two forms, knocking in genes or knocking out genes. Knocking out genes is by far the easier of the two. CRISPR targets the gene you want to silence, or knock out, and Cas9 will make a double strand cut in the DNA. The cells natural repair mechanism, called non-homologous end joining (NHEJ), the joins to the two cut ends together. This repair mechanism, however, is very imprecise and frequently introduces errors. Many of those errors will cause a shift in the genetic sequence that essentially ruins to code, effectively turning off the gene. This change is permanent, and will be carried to all later generations.
Knocking in a gene is more difficult. You not only have to make the cut at the desired location, you have to provide the genes sequence you want inserted and you need a different DNA repair mechanism called homology-directed repair (HDR), which is more precise and preserves the genetic sequence so that the gene remains active. But NHEJ is much more common than HDR, and so the trick is finding ways to enhance HDR repair so that a new gene can be successfully inserted at the repair site.
The new technique applies only to knocking out genes (no gene insertion), and in fact it does not alter the DNA sequence at all. The change is considered an epigenetic change for that reason. In this case CRISPR is paired with a different payload, which the published study says is a “single dead Cas9 fusion protein” and the result is called CRISPRoff. This system targets a specific gene as usual but then, instead of cutting the DNA, it methylates the gene – adding methyl groups to some of the base pairs. This typically makes it impossible for the transcriptase enzyme to work on the DNA at that location, so the gene cannot be transcribed and turned into protein. The gene is effectively off, even though its sequence has been preserved.
In the process of developing this technology the researchers made other interesting discoveries. The first is that this methylation carries through to subsequent generations, as far as they studied. They looked a pluripotent stem cells and turned them into neurons by turning off specific genes. The methylation persisted in the subsequent generations of the resulting neurons. This means that any gene silencing accomplished through CRISPRoff is “semipermanent”.
They also made a very surprising discovery – it was previously thought that this methylation mechanism would only work on certain genes that contained canonical CpG islands (CGIs), which is about a third of all genes. But the CRISPRoff technique worked on most genes studied, including those without CGIs. This means that our previous understanding of methylation was wrong in this regard, and also the CRISPRoff can be used on almost any gene. Both of these surprising discoveries increased the potential of CRISPRoff.
Finally the researchers developed CRISPRon, another system that takes away the methylation, turning the gene back on without any damage or alteration. Therefore researchers now have a relatively inexpensive and rapid method for reversibly turning genes off and back on, and is applicable to most genes. Turning off (or knocking out) a gene is the primary method that researchers use to investigate its function – turn it off and see what effect that has on the cell or the system being investigated. This is sure to be another boost to genetic research, perhaps as big as CRISPR itself. Genetic research has been progressing at a geometric, not linear, pace for the last half century at least. This is partly because our increased understanding of genetics improves the technology of genetic research itself, so there is a positive feedback loop. CRISPRoff is a great example of that.
This method probably won’t be used for creating GMOs, the goal of which is to change the DNA is a permanent and stable way – to make genetic changes, not epigenetic. But I could be wrong. Some GMOs are created only by silencing one or more genes, not by inserting any new genes. The fact that the changes are not permanent could even be an interesting method of patent protection. \
What about therapeutic applications? CRISPRoff would have incredible potential here. Anytime turning off a gene in a particular cell population could have a beneficial effects this technology could work. The one example brought up so far is Alzheimer’s disease. Part of the progression of this disease is caused by the build of of tau protein in neurons. If tau expression could be significantly turned down in brain cells, this might slow or even halt the progression of dementia.
The big challenge remains, however – how to target living cells in a whole organism? These techniques work great on cell cultures in a dish, but we don’t yet have a great mechanism to deliver CRISPR to targeted cell populations in a living creature. This is too big a topic to tackle today, so suffice to say there are several proposed methods all with potential. But none are great. They have problems getting to enough of the target cells, and not affecting off-target cell populations. So far using CRISPR for therapeutic uses involves taking cells out of the body, then altering them with CRISPR, then putting them back. This works on the blood or bone marrow, but would not work on the brain. Some tissues may be easier to target, like the retina. We could inject the CRISPR into the liquid inside the eye that is in contact with the retina, for example. Other organs are not so self-contained and would be difficult to target in this way. Perhaps the brain could be targeted through the cerebral spinal fluid (CSF)?
The CRISPR technology itself has advanced far more than therapeutic applications, partly because research on living people is more difficult and far more slow. The therapeutic potential for CRISPR remains to be seen, and may turn out to be more limited than we hope. But we’ll see.
Regardless, CRISPRoff is an amazing development that will at the very least become a powerful research tool.






