May 28 2024
A Greener Li-Ion Battery
 It is increasingly obvious that battery technology is one of the keys to transitioning our civilization away from burning fossil fuels. Batteries facilitate the use of cheap, green, but intermittent energy sources. They also allow for the electrification of technology sectors that are currently dominated by fossil fuel, such as transportation. There is nothing easy, however, about making fundamental changes to core technologies on an accelerated timescale. The task is pushing the limits of our resources and ingenuity, and there is predictable pushback from those most affected by the change.
It is increasingly obvious that battery technology is one of the keys to transitioning our civilization away from burning fossil fuels. Batteries facilitate the use of cheap, green, but intermittent energy sources. They also allow for the electrification of technology sectors that are currently dominated by fossil fuel, such as transportation. There is nothing easy, however, about making fundamental changes to core technologies on an accelerated timescale. The task is pushing the limits of our resources and ingenuity, and there is predictable pushback from those most affected by the change.
But there is some potentially good news – the relevant technologies are improving rapidly. In my opinion, the battery electric vehicle (BEV) is simply a superior technology to the internal combustion engine (ICE). Beyond the romantic cultural attachment to hum and roar of an engine, BEVs are more efficient, cheaper to operate, and virtually maintenance free. The fact that they are also better for the environment is almost incidental. The main challenge right now is infrastructure – installing all those charging stations.
It’s also pretty clear that the technology curve for BEVs is much steeper than for ICE vehicles (an already mature technology), so the technology advantage is likely to only increase. Core to BEV technology is the “B” part – batteries. The important stats of any BEV are largely driven by the battery – cost, range, recharge time, life expectancy. Right now the lithium-ion battery is the cutting edge technology, but there are lots of potential improvements on the horizon. There are essentially two types of possible battery technology advances – incremental advances in Li-Ion batteries themselves, or entirely new battery technology (like flow batteries). There are lots of potential new battery types being developed, but it seems that Li-Ion batteries likely have 1-2 decades of dominance left. There is also a lot of headroom in terms of perfecting the Li-Ion battery.
I already wrote about the next big change, swapping out the anode from graphite to silicon. This will give about a doubling of the battery energy density and specific energy (energy per volume and mass respectively). There may also be room to ultimately triple or quadruple Li-Ion battery capacity. Expect these batteries in BEVs in the next year or two.
The industry also has its sights on the cathode, which is currently made in most (but not all) Li-Ion batteries out of nickel and cobalt. These metals are expensive, and particularly with cobalt, the mining is plagued by environmental and human safety concerns. Right now the most common battery type has the following components:
By weight percentage (g material/g battery), a typical lithium-ion battery comprises about: 7% Co, 7% Li (expressed as lithium carbonate equivalent, 1 g of lithium = 5.17 g LCE), 4% Ni, 5% Mn, 10% Cu, 15% Al, 16% graphite, and 36% other materials.
Those percentages can be deceiving, however, because the cathode (where the cobalt and nickel are found) represent >50% of the cost of the battery. Another option, already in use, is iron phosphate cathode batteries, which are cobalt free. Right now about half of new Teslas use the iron phosphate batteries. The limitation here is that these batteries have a lower energy density, therefore a shorter range or a heavier battery. Essentially the lower range vehicles use the iron phosphate batteries, reserving the cobalt batteries for the longer ranged vehicles. In China they are moving toward sodium-ion batteries. Iron and sodium are abundant and cheap, so these batteries are less expensive, but again take a hit to energy density.
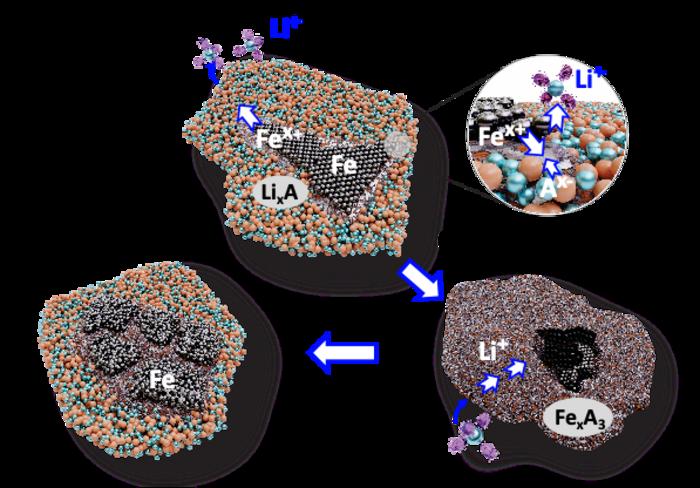
What we need is a better battery chemistry that allows for the replacement of cobalt and nickel with iron or sodium while potentially improving energy density (and maintaining or improving all the other necessary features of a good battery). There is a lot of research in this direction, and a new study shows there is some potential here. They found:
Our results show that Fe-cations and anions of F− and PO43− act as charge carriers in addition to Li-ions during the conversion from iron metal to a solid solution of iron salts. This composite electrode delivers a reversible capacity of up to 368 mAh/g and a specific energy of 940 Wh/kg.
For comparison, current Tesla batteries have a specific energy of 244 to 296 Wh/kg. The Amprius silicon anode battery has a specific energy of 450-500 Wh/kg. This approach is called a dual-ion battery, since both iron ion and lithium ions carry charge. The researcher also point out that potentially these batteries could be manufactured with existing infrastructure, and without a major change to manufacturing process.
However (you knew this was coming) the technology still has some kinks to work out. The storage efficiency is still limited – not all the energy that is stored in the battery is available for use. A high specific energy means nothing if you cannot access most of the energy. Study author Xiulei “David” Ji says that the industry needs to invest in further research into this approach to solve this one remaining problem. While this may be true, such statements always give me pause. Sometimes it works out – the same was said of the silicon anode 10 years ago, and a company did invest in the technology and work it out. Perhaps the same will be true of this approach to an iron-based cathode. But it doesn’t always work out – sometimes that last little problem becomes an unsolvable deal-killer.
But there are lots of labs and companies working on many potential battery advances. We only need some of them to work out. The net result is an impressive steady improvement in Li-ion battery technology, which should continue for at least another 2-3 decades, with significant improvement in specific energy. This will completely transform the BEV market.






