Feb 13 2024
Flow Batteries – Now With Nanofluids
 Battery technology has been advancing nicely over the last few decades, with a fairly predictable incremental increase in energy density, charging time, stability, and lifecycle. We now have lithium-ion batteries with a specific energy of 296 Wh/kg – these are in use in existing Teslas. This translates to BE vehicles with ranges from 250-350 miles per charge, depending on the vehicle. That is more than enough range for most users. Incremental advances continue, and every year we should expect newer Li-ion batteries with slightly better specs, which add up quickly over time. But still, range anxiety is a thing, and batteries with that range are heavy.
Battery technology has been advancing nicely over the last few decades, with a fairly predictable incremental increase in energy density, charging time, stability, and lifecycle. We now have lithium-ion batteries with a specific energy of 296 Wh/kg – these are in use in existing Teslas. This translates to BE vehicles with ranges from 250-350 miles per charge, depending on the vehicle. That is more than enough range for most users. Incremental advances continue, and every year we should expect newer Li-ion batteries with slightly better specs, which add up quickly over time. But still, range anxiety is a thing, and batteries with that range are heavy.
What would be nice is a shift to a new battery technology with a leap in performance. There are many battery technologies being developed that promise just that. We actually already have one, shifting from graphite anodes to silicon anodes in the Li-ion battery, with an increase in specific energy to 500 Wh/kg. Amprius is producing these batteries, currently for aviation but with plans to produce them for BEVs within a couple of years. Panasonic, who builds 10% of the world’s EV batteries and contracts with Tesla, is also working on a silocon anode battery and promises to have one in production soon. That is basically a doubling of battery capacity from the average in use today, and puts us on a path to further incremental advances. Silicon anode lithium-ion batteries should triple battery capacity over the next decade, while also making a more stable battery that uses less (or no – they are working on this too) rare earth elements and no cobalt. So even without any new battery breakthroughs, there is a very bright future for battery technology.
But of course, we want more. Battery technology is critical to our green energy future, so while we are tweaking Li-ion technology and getting the most out of that tech, companies are working to develop something to replace (or at least complement) Li-ion batteries. Here is a good overview of the best technologies being developed, which include sodium-ion, lithium-sulphur, lithium-metal, and solid state lithium-air batteries. As an aside, the reason lithium is a common element here is because it is the third-lightest element (after hydrogen and helium) and the first that can be used for this sort of battery chemistry. Sodium is right below lithium on the period table, so it is the next lightest element with similar chemistry.
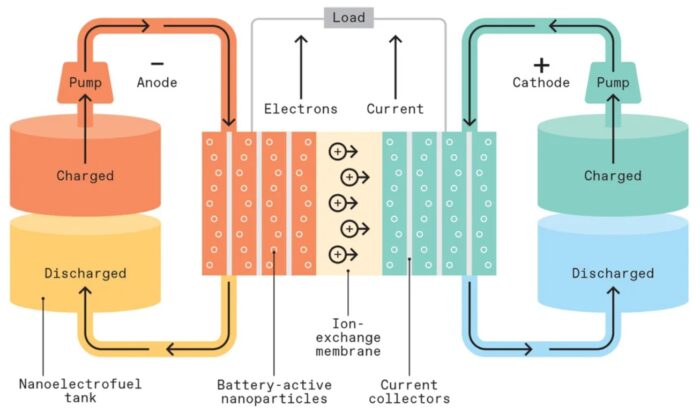
But for the rest of this article I want to focus on one potential successor to Li-ion batteries – flow batteries. So-called flow batteries are called that because they use two liquid electrochemical substance to carry their charge and create electrical current. Flow batteries are stable, less prone to fires than lithium batteries, and have a potential critical advantage – they can be recharged by swapping out the electrolyte. They can also be recharged in the conventional way, by plugging them in. So theoretically a flow battery could provide the same BEV experience as a current Li-ion battery, but with an added option. For “fast charging” you could pull into a station, connect a hose to your car, and swap out spent electrolyte for fresh electrolyte, fully charging your vehicle in the same time it would take to fill up a tank. This is the best of both worlds – for those who own their own off-street parking space (82% of Americans) routine charging at home is super convenient. But for longer trips, the option to just “fill the tank” is great.
But there is a problem. As I have outlined previously, battery technology is one of those tricky technologies that requires a suite of characteristics in order to be functional, and any one falling short is a deal-killer. For flow batteries the problem is that their energy density is only about 10% that of Li-ion batteries. This makes them unsuitable for BEVs. This is also an inherent limitation of chemistry – you can only dissolve so much solute in a liquid. However, as you likely have guessed based upon my headline, there is also a solution to this limitation – nanofluids. Nanoparticles suspended in a fluid can potentially have much greater energy density.
Research into this approach actually goes back to 2009, at Argonne National Laboratory and the Illinois Institute of Technology, who did the initial proof of concept. Then in 2013 DARPA-energy gave a grant to the same team to build a working prototype, which they did. Those same researchers then spun off a private company, Influit Energy, to develop a commercial product, with further government contracts for such development. As an aside, we see here an example of how academic researchers, government funding, and private industry work together to bring new cutting edge technology to market. It can be a fruitful arrangement, as long as the private companies give back to the public the public support they built upon.
Where is this technology now? John Katsoudas, a founder and chief executive of Influit, claims that they are developing a battery with an specific energy of 550 to 850 Wh/kg, with the potential to go even higher. That’s roughly double to triple current EV batteries. They also claim these batteries (soup to nuts) will be cost competitive to Li-ion batteries. Of course, claims from company executives always need to be taken with a huge grain of salt, and I don’t get too excited until a product is actually in production, but this does all look very promising.
Part of the technology involved how much nanoparticles they can cram into their electrolyte fluid. They claim they are currently up to 50% by weight, but believe they can push that to 80%. At 80% nanoparticles, the fluid would have the viscosity of motor oil.
A big part of any new technology, often neglected in the hype, is infrastructure. We are facing this issue with BEVs. The technology is great, but we need an infrastructure of charging stations. They are being built, but currently are a limiting factor to public acceptance of the technology (lack of chargers contributes to range anxiety). The same issue would exist with nanoparticle flow batteries. However, they would have at least a good an infrastructure for normal recharging as current BEVs. Plus also they would benefit from pumping electrolyte fluid as a means of fast charging. Such fluid could be process and recharged on site, but also could be trucked or piped as with existing gasoline infrastructure. Still, this is not like flipping a switch. It could take a decade to build out an adequate infrastructure. But again, meanwhile at least such batteries can be charges as normal.
I don’t know if this battery technology will be the one to displace lithium-ion batteries. A lot will depend on which technologies make it to market first, and what infrastructure investments we make. It’s possible that the silicon anode Li-ion batteries may improve so quickly they will eclipse their competitors. Or the solid state batteries may make a big enough leap to crush the competition. Or companies may decide that pumping fluid is the path to public acceptance and go all-in on flow batteries. It’s a good problem to have, and will be fascinating to watch this technology race unfold.
The only prediction that seems certain is that battery technology is advancing quickly, and by the 2030s we should have batteries for electric vehicles with 2-3 times the energy density and specific energy of those in common use today. That will be a different world for BEVs.






