May 24 2024
MOBE – A New Gene Editing System
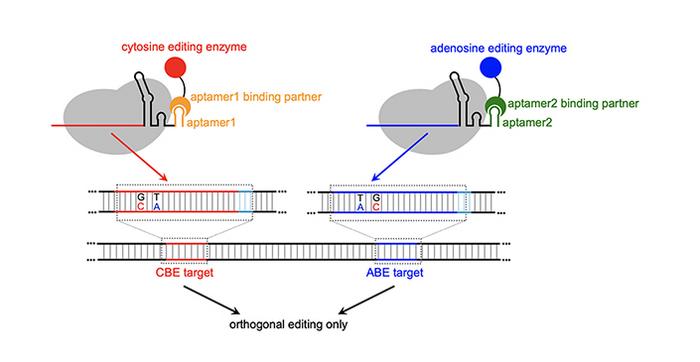 Have you memorized yet what CRISPR stands for – clustered regularly interspaced short palindromic repeats? Well, now you can add MOBE to the list – multiplexed orthogonal base editor. Base editors are not new, they are basically enzymes that will change one base – C (cytosine), T (thymine), G (guanine), A (adenosine) – in DNA to another one, so a C to a T or a G to an A. MOBE is a guided system for making multiple desired base edits at once.
Have you memorized yet what CRISPR stands for – clustered regularly interspaced short palindromic repeats? Well, now you can add MOBE to the list – multiplexed orthogonal base editor. Base editors are not new, they are basically enzymes that will change one base – C (cytosine), T (thymine), G (guanine), A (adenosine) – in DNA to another one, so a C to a T or a G to an A. MOBE is a guided system for making multiple desired base edits at once.
This is a complementary system to CRISPR, which targets a sequence of DNA and then uses Cas9 or a similar payload to make a double-stranded cut in the DNA. The cells natural repair system can then be leveraged to make changes during the repair process, such as inserting a new genetic sequence. In this way, and with different payloads, CRISPR can make targeted gene insertions or deletions, kill targeted cell types, or turn genes off and back on again.
MOBE cannot insert entire genes. Rather, systems like this can make single base edits. What is new about the MOBE system is that it can make multiple different types of edits at once. Some single base edits can change the nature of the resulting protein. Many single base changes in DNA are “silent” meaning that they do not alter the resulting amino acid that is coded for, because each amino acid has 3-4 similar three base pair codes. It’s also possible that a single base mutation will change the amino acid coded for, but the new amino acid is structurally similar to the previous one, so no conformational change in the protein results. But some point mutations will change one amino acid for a different one with a different effect – turning a straight line into a kink, for example. These alter the three dimensional folded structure of the protein, and therefore its function. Some point mutations may also change the code to what is called a stop codon, ending the production of the protein at that point and dramatically changing its structure.
This is the basis for many genetic diseases, they are point mutations. Some may be the result of a single point mutation, while others result from a combination of point mutations. The primary initial purpose of MOBE is to alter a cell line in culture with multiple different but specific point mutations in order to model a genetic illness. This allows for the creation on demand of biological models of these genetic diseases, and is therefore a boon to research.
The system uses guide RNA to target the desired location on the DNA. They combine these with RNA aptamers, which are small loops of RNA that bind to, and therefore can recruit, specific proteins. They use aptamers which recruit enzymes that make specific base edits – ABEs (adenosine base editors) in combination with CBEs (cytosine base editors). The RNA guides determine where to make the change, and the aptamers determine what change to make.
The main advantage to the MOBE system to simply performing multiple individual base edits is that this system reduces “crosstalk”, which refers to making base edits at undesired locations. Individual changes result is crosstalk about 30% of the time. With MOBE this is reduced to about 5%, while the rate of successfully making the desired changes is 30%. This is a more efficient and accurate system than what’s currently in use.
The main purpose of MOBE is research, and this level of efficiency is fine for conducting genetic research on cells in culture. But of course, for any gene editing system, there is always the question – can this be used therapeutically? We now have, for example, the first FDA approved CRISPR treatments, for sickle cell disease and thalassemia. Is MOBE accurate enough for a therapeutic intervention? That is yet to be determined, along with the related question, how much potential is there to improve the accuracy of MOBE? What threshold do we need to get to in order for the benefits to outweigh the risks? That likely depends on the application.
As I pointed out in the linked post, sickle cell and thalassemia are the lowest of hanging fruit for a therapeutic gene-editing application. This is because we can take cells out of the patient (from bone marrow), then treat them with CRISPR, ensure that we have viable healthy cells, and then transplant them back into the patient. MOBE can theoretically be used in a similar fashion, where one or more point mutations are desired to correct or compensate for a genetic illness.
These gene-editing tools can also be used theoretically on in-vitro fertilized eggs. Candidate embryos from parents where one or both have known severe genetic disease or are carriers can be treated prior to implantation. This can prevent the disease from being transmitted to the child, or even just assure that they are not a carrier. In vitro embryo gene therapy will take much longer, not because it’s harder to do, but because the risk if higher from unwanted crosstalk changes. When altering bone marrow cells, those changes affect only the one patient. But when changes are made to a genome in an embryo, that affects the entire person, and can be passed down to future generation. So those changes are being made to the human gene-pool, not just to an individual. This requires a much higher level of safety testing.
A third type of application is to somatic cells in living people, not in embryos, and not in cells that can be removed from the body. The challenge here is getting the gene editing tool (CRISPR or MOBE) to the desired cell population. Let’s say you want to edit muscle cells to treat a muscular dystrophy. How do you get the gene-editing tool to enough of the muscle cells to have a clinical impact? Right now we use mostly viral vectors, which are effective but risky. Many viruses evolved to infect a specific cell type and deliver their own genetic payload. We can therefore select and alter specific viruses to target the cells we want, and to deliver our own payload – such as CRISPR. The problem is that viruses have a nasty habit of causing infections – they are not always compliant little workers.
There have been recent advances in viral vector technology. They work, and there is the potential for continued incremental advances. It’s still hard to predict how long it will be before we have an approved viral vector-based therapy (using CRISPR or something else, perhaps MOBE) for a genetic illness. There is a lot of research being done, but proving safety is a high bar.
The research is advancing quickly. It still feels like we are on the steep part of the curve when it comes to genetic technology. Significant advances are being made at a rapid pace, and MOBE is just one more example of this progress.






