Jul 02 2020
Homeopathy is Impazable
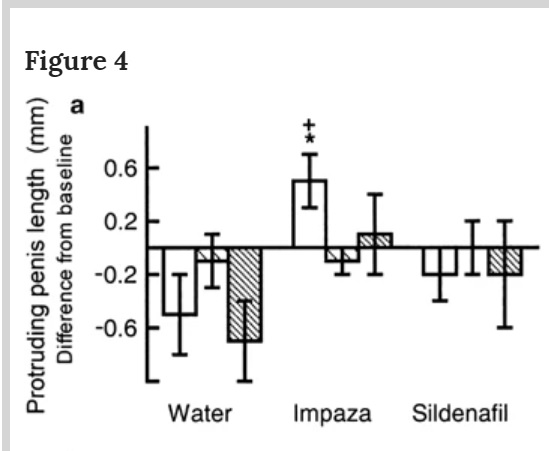 A study by Russian researcher purports to find that a treatment, Impaza, increases penis length during copulation in rats, while the water placebo group and sildenafil (Viagra) did not. The authors conclude: “This effect, together with an absence of motivational actions, suggests that Impaza may be the most valuable treatment for erectile dysfunction.” The study was originally published in the International Journal of Impotence Research, but was later retracted by the editors. The primary reason for the retraction is that the editors discovered that Impaza is a homeopathic product, something that was apparently missed on initial peer review.
A study by Russian researcher purports to find that a treatment, Impaza, increases penis length during copulation in rats, while the water placebo group and sildenafil (Viagra) did not. The authors conclude: “This effect, together with an absence of motivational actions, suggests that Impaza may be the most valuable treatment for erectile dysfunction.” The study was originally published in the International Journal of Impotence Research, but was later retracted by the editors. The primary reason for the retraction is that the editors discovered that Impaza is a homeopathic product, something that was apparently missed on initial peer review.
This is definitely an editorial fail, but at least it was quickly corrected. To put the failure in context, however, Impaza was not presented as homeopathic, but rather as a “release-active antibody-based” drug. This is code in Russia, apparently, to disguise the homeopathic nature of certain products. It is not uncommon in pseudoscience for proponents to come up with scientific sounding euphemisms for their nonsense in order to hide from the negative association with charlatans and quacks.
Editors and reviewers, however, need to dig deep enough to uncover such pseudoscience. At the very least there was a lack of curiosity on the part of the editors, and insufficient vigilance against the intrusion of pseudoscience.
But let’s explore a slightly deeper issue here – should the paper have been retracted purely on the basis that the treatment being studied was homeopathic? The editors write:
“The editor has retracted this article because there are concerns about the scientific validity of the study. Specifically, the reagent is diluted beyond the point to which any active molecules are expected to be present and there is no molecular analysis to support the presence of molecules at these dilutions. These concerns have caused the editor to lose faith in the reliability of the findings.”
In other words, the treatment arm of the study was just water, and the researchers did not adequately demonstrate otherwise. Therefore the results must be bogus. This is where proponents of magic medicine often call foul. They say this is rigging the game against things like homeopathy, and how will they ever prove it works if they cannot get their research published because the scientific community is already convinced it doesn’t work?
This position, however, misses a few points, most importantly the role that clinical research plays in medicine. First, clinical research is not the only kind of research. There is also basic science and pre-clinical research. These different types of research build on each other and inform each other. Basic science may provide a plausible mechanism for clinical research to then test whether or not it is safe and effective. But also clinical research may show a potential treatment is effective, leading back to basic science to figure out the mechanism.
Further it is important to realize that all types of research have their weaknesses. Clinical research is often very difficult because outcomes can be subjective, or they can be difficult to measure, and they can also be highly variable because creatures (including people) are highly variable. It is difficult, therefore, for clinical research to be so solid in its conclusions that it overrides conclusions about plausibility based on basic science.
But this point requires further explanation – this depends highly on what we mean by “plausible.” We may make our best guess about what is likely to happen in a complex system based upon what we know, and clinical research may show that guess to be wrong. This situation, however, cannot be treated the same as one in which a proposed treatment is so implausible that in order for it to work we would have to break the laws of physics, or fundamentally rearrange our knowledge of chemistry, physiology, or biology. Homeopathy is in this later category.
In the retraction statement the editors hit upon the key logic:
“…these innovative “drugs” contain no active molecules and can be considered a new brand of homeopathy. This indicates one of two possibilities: either we are at the brink of a revolution in medicine or that something went wrong with research published in numerous academic journals. We argue that the latter explanation is more likely and that this conclusion has severe implications for the scientific and healthcare enterprises.”
That is what it comes down to. When basic science and clinical research disagree, we can apply Occam’s razor to determine which one is likely wrong. Sometimes it’s a tie, and we just need to do further research. Sometimes the basic science is uncertain, and the clinical data fairly solid. And sometimes the basic science is solid and the clinic research dubious. These situations should not be treated all the same.
In the case of homeopathy we have basic science principles from physics, chemistry, and biology that all lead to the unavoidable conclusion that the claims of homeopathy at all but impossible, and should be treated as such. What does this mean about the clinical research? Standard medical scientific logic dictates that it means clinical research is unethical. You are giving subjects an intervention that cannot possibly help them. Animal research, as in this study, is not quite as unethical (there is a lower threshold) but still we want to use research animals with respect.
If someone wants to study homeopathy, therefore, they should be doing basic science research to determine whether or not there is a plausible mechanism. Demonstrate that before you do clinical research. They cannot, however, because homeopathy is actually impossible. Clinical research is very easy to manipulate (see my articles on p-hacking) so it is easier to ignore the basic science obstacles and just keep putting out crappy clinical research.
I should point out that in this study, as with much of homeopathic research, the data is suspect independent of the homeopathic nature of the treatment. The primary outcome, penis length, was estimated from video, and the authors admit they often could not get a good estimate. Any subjectivity in measuring a primary outcome makes me nervous. The paper claims all measurements were made by an assistant blind to the treatment, but that is crucial. Were they really blind?
Look at the graph above. The placebo group, that just received water, had a significant decrease in penis length. Why? That is a huge red flag – when the placebo group does worse than would be expected by history or first principles. The water should have had no effect. Might the subjective estimates of the “blinded” assistant been not so blind? This would not be the first time this has happened in homeopathy research.
At the very least, studying an alleged treatment that appears to be scientifically implausible, even impossible, should carry a significantly higher burden of proof. It is reasonable to conclude that this study does not meet that burden and should not have been published. I would also argue that when the study is of a treatment or product that is on the market and is likely defrauding the health of the public, special care must be taken. The editors did not initially take that care, but at least they corrected their mistake.






