Feb 08 2022
Incremental Advance Treating Spinal Cord Injury
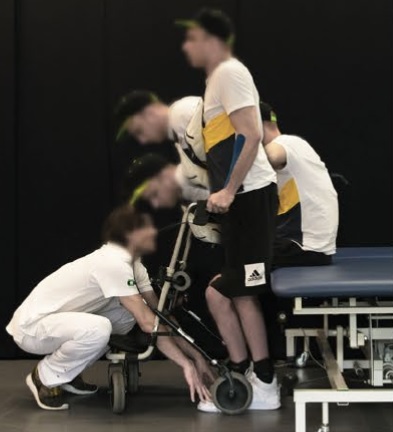 In my opinion one of the most encouraging future technologies that we are developing today is hacking the nervous system through electromagnetic recording and stimulation. Nervous system signals are ultimately electrical, which is convenient because we have an entire mature technology based on controlling the flow of electricity. We also have increasingly powerful computers and software algorithms to both read and recreate these electrical signals. The actual limiting factor with this technology at present is the hardware – the electrodes we use to interface with nervous tissue. As this technology advances, so do our applications.
In my opinion one of the most encouraging future technologies that we are developing today is hacking the nervous system through electromagnetic recording and stimulation. Nervous system signals are ultimately electrical, which is convenient because we have an entire mature technology based on controlling the flow of electricity. We also have increasingly powerful computers and software algorithms to both read and recreate these electrical signals. The actual limiting factor with this technology at present is the hardware – the electrodes we use to interface with nervous tissue. As this technology advances, so do our applications.
That is exactly the incremental advance that is now being reported, with specific reference to severe spinal cord injury. However, I think that much of the mainstream reporting is missing some important details. First let’s discuss the actual science – published in Nature is a report on three proof-of-concept cases using spinal cord stimulation to allow those with spinal cord injury to walk. This is old technology, which has been developed over the last several decades, called EES – Epidural Electrical Stimulation. So it is nothing new. The report is about a small but meaningful improvement in the hardware.
The three subjects all had spinal cord injury with complete motor and sensory loss below the level of the injury, which means essentially that they could not move or feel their legs at all. The technology implants electrodes on the dorsal roots below the injury. These are are the trunks of spinal nerves that deliver sensory information to the spinal cord. Why would you stimulate sensory nerves to make the muscles move? Because those signals get into the spinal cord (which is a physically small space) where they stimulate motor neurons. Neuroscientists have learned how to arrange these electrodes to guide these electrical signals to where they want, and keep them from spreading to motor neurons on the other side or ones they don’t want to stimulate. By taking this route they are able to stimulate a group of motor neurons that are typically involved on a motor task, like walking. If they instead stimulated the ventral motor root that would just make the entire leg contract, without having it move in a useful way.
The innovation here is a change in the electrodes used. Previously the neuroscientists uses electrodes that were designed to stimulate the dorsal roots to relieve pain. These electrodes were repurposed for EES, but they were not designed for the purpose and not ideal. They are, for example, too short to get to all of the desired stimulation points. So the researchers designed new electrodes to more precisely stimulate the dorsal roots in the desired pattern. This improvement worked, allowing the subjects to walk using this external stimulation. Also, they were able to walk much more quickly, with less training, than the older technology.
That’s the advance. It’s nice, but it is very incremental. But of course the media has to parlay this into as dramatic an advance as possible. The BBC incorrectly says, for example, that this is the first time a subject with a completely severed spinal cord has been able to walk. These subjects did not even have a completely severed spinal cord. I think the reporter confused complete paralysis with complete severing. The reporting does correctly state that the technology is not a cure for the injury and is not used routinely in everyday life. But still I think they give the wrong impression of how it works.
Their diagram and explanation says that the implant “boosts the signal” from above the injury. Of course, that would be incompatible with a completely severed cord, so they contradict themselves. The stimulator is entirely below the injury, and is activated by external control, not by signals from the brain down the spinal cord. However, the reporter is sort-of correct about “boosting the signal” above the injury. I say sort-of, because the details are different. Again, the activation signals are completely independent of anything happening above the injury. What the researchers did find is that with practice the subjects were able to modulate the activity of their leg muscles being generated by the external stimulation. Voluntary modulation implies that some spinal cord signal is getting through the injury point. Further, after using the device for a while they were able to regain very slight voluntary movement of their legs.
The researchers interpret this to mean that there were some residual neurons that were not completely killed by the injury (and therefore the cords could not, by definition, have been completely severed). These residual motor neurons were asleep because of lack of stimulation, and the EES device reactivated them. But again, this produced only slight motor control, not enough by itself to stand, walk, or do anything meaningful. But it did add a small bit of voluntary control to the movements generated by the EES, and that might be clinically relevant.
It is unclear if this technological approach to spinal injury will lead to any meaningful recovery that would be relevant in everyday life. Rather, it appears to be a useful rehab tool, allowing patients to move and exercise, which has some physiological benefits, such as better blood pressure. Perhaps the mature version of this technology might be good enough to allow people to walk freely, almost normally. I suspect that will require more than a stimulator, such as the addition of some robotic assist.
This approach does not address some of the problems brought about by severe spinal cord injury. The lack of signals from above the injury causes severe spasticity in the legs, for example. This can be treated, but suppressing the spasticity also causes muscle weakness, and the muscles are already weak from lack of use. Lack of sensory input is also a problem, as that is essentially to natural-feeling control of the legs.
A far better approach, if we can make it work, is to biologically regenerate the spinal cord, restoring sufficient activity across the injury to restore motor and sensory function. This is a completely different approach, and researchers have been working on it also for decades. We have made significant basic science progress, but are not yet at the point where we can functionally regenerate a spinal cord, or even close enough that we can accurately guess how long it will take to cross that threshold. Perhaps a combination of the two approaches might work best – restoring some spinal cord function, but then augmenting that function with some external control. The next few decades should prove interesting.






