May 02 2024
Understanding Jumbo Phage Viruses
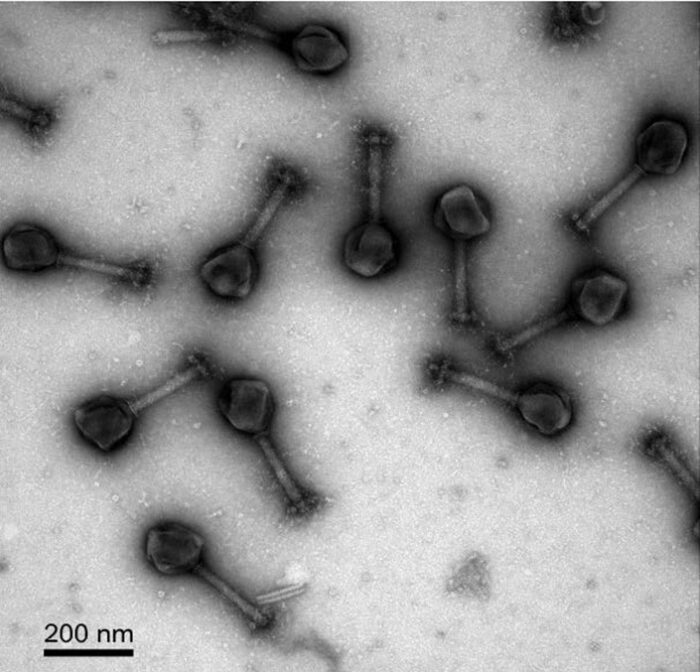 Bacteriophages, viruses that infect bacteria, are the most abundant form of life on Earth. And yet we know comparatively little about them. But in recent years phage research has taken off with renewed interest. This is partly driven by the availability of CRISPR-based tools for studying genomes. Interestingly, CRISPR itself is a gene-editing tool that derives from bacteria and archaea, which evolved the system as a defense against viruses that infect them and alter their genome. Now we are using CRISPR to investigate those very viruses, and perhaps use that knowledge as a tool to fight bacterial infections. Bacteria may have handed us the tools to fight bacteria.
Bacteriophages, viruses that infect bacteria, are the most abundant form of life on Earth. And yet we know comparatively little about them. But in recent years phage research has taken off with renewed interest. This is partly driven by the availability of CRISPR-based tools for studying genomes. Interestingly, CRISPR itself is a gene-editing tool that derives from bacteria and archaea, which evolved the system as a defense against viruses that infect them and alter their genome. Now we are using CRISPR to investigate those very viruses, and perhaps use that knowledge as a tool to fight bacterial infections. Bacteria may have handed us the tools to fight bacteria.
Most phage viruses are small, with genomes smaller than 200 kbp (kilo-base pairs). But a very few (93 so far) are larger than this, and known as jumbo phage viruses. The largest of these, Bacillus megaterium, is 497 kbp, which is only 87 kbp smaller than the smallest known bacterium, Mycoplasma genitalium. So essentially these are viruses that are almost as big as bacteria.
The jumbo phage viruses have been especially difficult to study for various technical reasons. For one, the filters that separate viruses from bacteria tend to trap the jumbo phages also. The genome has also been difficult to get access to. But CRISPR is changing that, giving us new tools to investigate these viruses. Researchers have recently published some interesting findings. When some jumbo viruses infect a bacterium they form a pseudonucleus that functions similar to the nucleus in eukaryotic cells, meaning that it is a walled-off section within the bacteria containing the viral genome. The purpose of forming this viral nucleus is to protect the genome from the bacterium, which will try to destroy or disable it before it can replicate.
This is part of what the researchers were studying – how does the jumbo phage virus protect its nucleus inside a bacterium. They have identified a key protein, “protein importer of Chimalliviruses A,” or PicA, that acts as a chaperone. It will allow useful cargo proteins across the nucleus barrier but not hostile bacterial proteins. It’s a mini-transport system, the smallest biological one known. Because jumbo phage viruses are larger than most phage viruses they have more genes which allow for more complex behavior, including setting up this nucleus and transport system.
Interest in jumbo phage viruses is more than academic. There are potentially huge applications. Downstream applications are often hard to predict, and usually take longer than we would like. I have found a good rule of thumb is that there is about 30 years between such basic-science discoveries and clinical applications. This time frame may be decreasing, however, as the pace of biological research accelerates. CRISPR and AI technology in particular have increased the speed of this type of research considerably. Translating to clinical applications, however, still takes a long time because of the safeguards in animal and human research.
Researchers are eyeing one particular application, which I referred to above – using phage viruses (jumbo or otherwise) to fight bacterial infections. Right now we are facing a real threat of antimicrobial resistance. Those pesky infectious bacteria won’t just let us kill them. They stubbornly insist on evolving ways to bypass and survive our antibiotics, known as antibiotic (or more broadly, antimicrobial) resistance. Antimicrobial resistance is already responsible for millions of deaths per year, and is a growing problem. Researchers are trying to develop new drugs with novel mechanisms of killing bacteria, but the concern is that this is ultimately a losing battle. Evolution will eventually win out.
As an aside, I think this remains to be seen. Part of the problem is overprescribing and misusing of antibiotics. Better regulation and enforcement can help, slowing the development of resistance. But this can only slow resistance, not stop it. Researching novel mechanisms also helps, but again this just kicks the can down the road. It will be interesting to see how this will ultimately play out. Can we develop enough different kinds of antibiotics that we can essentially use them in a rotation, taking certain drugs entirely off the market for a couple of decades to allow bacteria to lose resistance? Will that even work? And how quickly will they regain resistance once the antibiotics are reintroduced?
This is why there is so much interest in bacteriophages. They could provide not just another pharmaceutical option, but an entirely different approach to fighting clinical bacterial infections. Phages have been engaged in an evolutionary battle with bacteria for perhaps over a billion years, so we have a lot to learn from them. We could, theoretically, genetically engineer a bacteriophage to efficiently and safely infect and kill an infectious bacteria. Such viruses would likely not infect eukaryotic cells.
Another possibility is to use the bacteriophages, especially the jumbo phages, as drug-delivery systems. They target and get inside bacteria. Once there they could be engineered to then release an antibiotic, one that would not have been able to otherwise gain access to the bacteria. Or this approach could simply make antibiotics much more effective. This could also reduce the side effects and risks of antibiotics as they will be precisely delivered.
Phages can also have applications beyond fighting bacteria. They can be engineered and selected for specificity against cancer cells. These anti-cancer phages can then, again, be used to deliver drugs to their target, this time chemotherapy rather than antibiotics.
This all seems like a useful technology to develop. The more we learn about the basic science of bacteriophages, the better we will be able to then manipulate them for our own applications.






