Aug 10 2023
The Alzheimer’s Revolution
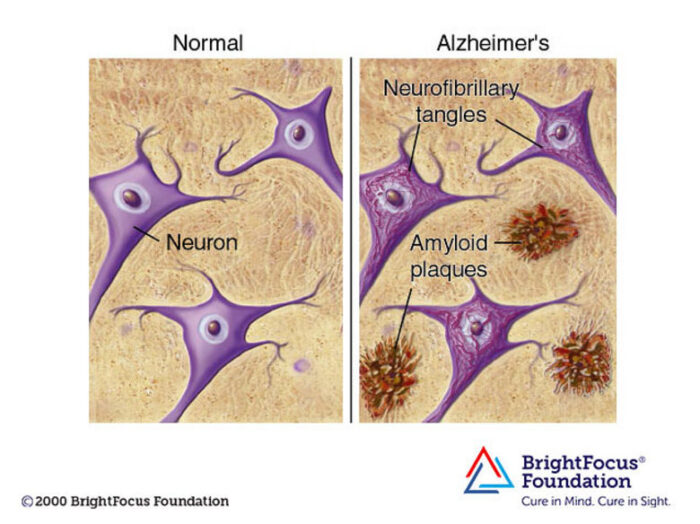 Decades of complex research and persevering through repeated disappointment appears to be finally paying off for the diagnosis and treatment of Alzheimer’s disease (AD). In 2021 Aduhelm was the first drug approved by the FDA (granted contingent accelerated approval) that is potentially disease-modifying in AD. This year two additional drugs received FDA approval. All three drugs are monoclonal antibodies that target amyloid protein. They each seem to have overall modest clinical effect, but they are the first drugs to actually slow down progression of AD, which represents important confirmation of the amyloid hypothesis. Until now attempts at slowing down the disease by targeting amyloid have failed.
Decades of complex research and persevering through repeated disappointment appears to be finally paying off for the diagnosis and treatment of Alzheimer’s disease (AD). In 2021 Aduhelm was the first drug approved by the FDA (granted contingent accelerated approval) that is potentially disease-modifying in AD. This year two additional drugs received FDA approval. All three drugs are monoclonal antibodies that target amyloid protein. They each seem to have overall modest clinical effect, but they are the first drugs to actually slow down progression of AD, which represents important confirmation of the amyloid hypothesis. Until now attempts at slowing down the disease by targeting amyloid have failed.
Three drugs in as many years is no coincidence – this is the result of decades of research into a very complex disease, combined with monoclonal antibody technology coming into its own as a therapeutic option. AD is a form of dementia, a chronic degenerative disease of the brain that causes the slow loss of cognitive function and memory over years. There are over 6 million people in the US alone with AD, and it represents a massive health care burden. More than 10% of the population over 65 have AD.
The probable reason we have rapidly crossed over the threshold to detectable clinical effect is attributed by experts to two main factors – treating people earlier in the disease, and giving a more aggressive treatment (essentially pushing dosing to a higher level). The higher dosing comes with a downside of significant side effects, including brain swelling and bleeding. But that it what it took to show even a modest clinical benefit. But the fact that three drugs, which target different aspects of amyloid protein, show promising or demonstrated clinical benefit helps confirm that the amyloid protein and the plaques they form in the brain are, to some extend driving AD. They are not just a marker for brain cell damage, they are at least partly responsible for that damage. Until now, this was not clear.
But there is more than the build up of abnormal amyloid going on in the brains of those with AD. The other major abnormal protein is tau, which forms tangles. Plaques and tangles are pathological markers for AD (and to some extend normal aging). Genetic variants for these proteins predict risk of developing AD, and now there are spinal fluid, blood, and imaging tests based on detecting types of these proteins to help confirm the diagnosis of AD. Diagnosis and treatment go hand-in-hand, as early diagnosis (with mild symptoms, and eventually even before symptoms emerge) is critical to the effectiveness of treatment.
Of the two, amyloid and tau, tau seems to correlate better with the rate of disease progression. This is why some researchers, until now, still held the belief that amyloid might be a dead end. Perhaps we should focus on treatments that target tau. It seems likely now that both proteins are involved, opening the door for combination therapies that might be more effective than targeting either protein alone. But treatment, as I said, starts with diagnosis.
One of the diagnostic modalities for AD is PET scanning – positron emission tomography. PET scanning detects positrons from specific radioactive tracers that target something biological of interest. The classic application is to label glucose and than use that to image glucose metabolism in the brain. The brain is a hungry organ and uses glucose for energy, so glucose metabolism is a great marker for brain activity. The result is color-coded images of the brain showing relative brain activity, useful for diagnosing brain diseases that might either increase (such as with tumors) or decrease (such as with stroke) brain activity. This is not as fast as functional MRI scanning, so is not useful for real-time imaging (during a specific task, for example) but is useful for imaging disease states.
Tracers, however, can target things other than glucose, and for AD the two obvious targets are amyloid and tau. How do these various PET imaging techniques fair as predictors of the disease? A recent study compares them head-to-head. They found:
Metabolism had the strongest association with cognition (r = 0.712; p < 0.001), followed by tau (r = -0.682; p < 0.001). Neocortical tau showed the strongest association with cognitive decline (r = -0.677; p < 0.001). Metabolism mediated the association between tau and cognition and marginally mediated the one with decline. Tau positivity represented the strongest risk factor for decline (hazard ratio = 32).
What this means is that glucose metabolism was the best overall indicator of the presence of AD, because this measures overall brain function. Both the presence of tau and amyloid also correlated with decreased cognitive function, but of the two tau had a stronger correlation. When looking at decline over time, of the three markers tau had the strongest correlation with decline. This suggests that tau deposition, at least in most AD patients, may be more of a factor in driving neurodegeneration than amyloid.
However, targeting tau proteins in AD has so far been disappointing. Keep in mind that both amyloid and tau are normal structural proteins in the brain, but in AD there are abnormal forms of these proteins that cause them to clump together into plaques and tangles. There are many forms of these proteins, and different active regions and regulators. So just saying “targeting tau” is not sufficient. To give you a flavor of the complexity, here is a summary of treatments tried so far:
More than 30 drugs have reached the clinic including two tau aggregation inhibitors, three microtubule stabilizers, three glycogen synthase kinase-3β inhibitors, one tau acetylation inhibitor, three O-GlcNAcase inhibitors, two anti-tau active vaccines, 11 anti-tau monoclonal antibodies, one tau antisense oligonucleotide, and one progranulin enhancer.
None of these have worked. But, as with amyloid, researchers are not giving up. They have moved on to other regions of tau and are progressing with new monoclonal antibody studies targeting these new regions. Having success with amyloid after thirty years of similar failure shows how tenacious AD researchers are, and certainly is good reason to keep going with tau. This shows how tedious and detailed scientific research can be, especially with a disease as complex as AD.
But many researchers think we are seeing the light at the end of the tunnel. The hope is that these newer diagnostic tests will allow for early detection of AD, even before symptoms appear or in the mildest stages. Further, some combination of amyloid and tau treatments, a cocktail targeting multiple protein regions, will significantly slow progression of the disease, to the point that symptoms may not become significant over a typical lifespan. It may even be possible, by clearing proteins that have already accumulated, to partially reverse existing damage. Now that we have shown disease-modifying efficacy for some treatments, there is reason to hope we are on track for incremental advances that will slowly but surely bring AD into the realm of a manageable chronic illness.






