Oct 13 2023
Liquid Organic Hydrogen Carriers as Fuel
 The press release for a recent study declares: “New catalyst could provide liquid hydrogen fuel of the future.” But don’t get excited – the optimism is more than a bit gratuitous. I have written about hydrogen fuel before, and the reasons I am not optimistic about hydrogen as a fuel for extensive use in transportation. Nothing in the new research changes any of this – most hydrogen production today uses fossil fuels and is worse than just burning the fossil fuel, hydrogen does not have very good energy density, and requires an infrastructure not only for manufacture but storage and transportation. It’s also a very leaky and reactive molecule, so challenging to deal with. It may see a future in some niche applications, but for cars it is progressively losing the competition with battery electric vehicles.
The press release for a recent study declares: “New catalyst could provide liquid hydrogen fuel of the future.” But don’t get excited – the optimism is more than a bit gratuitous. I have written about hydrogen fuel before, and the reasons I am not optimistic about hydrogen as a fuel for extensive use in transportation. Nothing in the new research changes any of this – most hydrogen production today uses fossil fuels and is worse than just burning the fossil fuel, hydrogen does not have very good energy density, and requires an infrastructure not only for manufacture but storage and transportation. It’s also a very leaky and reactive molecule, so challenging to deal with. It may see a future in some niche applications, but for cars it is progressively losing the competition with battery electric vehicles.
This new study does not alter the basic situation. But it does raise the potential of one way to deal with hydrogen, potentially for one of those niche applications. The biggest challenge for “the coming hydrogen economy” (which never came) is storage. There are basically three choices for storing hydrogen for use in a hydrogen fuel cell. You can cool it to liquid temperatures, you can compress it as a gas, or you can bind it up in some other material.
Hydrogen is the lightest element, and it contains a lot of potential energy, and for those reasons is an excellent fuel. It is the best fuel, arguably, for rockets, because it has the greatest specific energy (energy per mass), and for the rocket equation, energy per mass is everything. We may never do better than pure hydrogen as rocket fuel. Liquid hydrogen has about three times the energy per mass as gasoline. So theoretically it might seem like a good fuel. But – it has only one third the energy density (energy per volume) of gasoline. This is a limiting factor for small mobile applications like cars. Imagine a 50 gallon tank of liquid hydrogen to go as far as a 17 gallon tank of gasoline. Also, liquid hydrogen has to be kept very cold.
Compressed hydrogen gas has even worse energy density. It has about two thirds the energy density of liquid hydrogen, even at high pressures. And of course you are trading low temperatures for very high pressure, which has its own technical challenges and risks.
Two decades ago, when people were still talking about the hydrogen economy, the belief (hope) was that scientists would develop a hydrogen storage medium. There was a lot of research done with ceramics that could hold a lot of hydrogen at ambient pressures and temperatures and release it quickly. But we were never able to get to an accepted specific energy – the ceramics were simply too heavy for the amount of hydrogen they can hold. That roadblock stalled the deployment of hydrogen fuel cell cars, allowing for BEVs to take the lead.
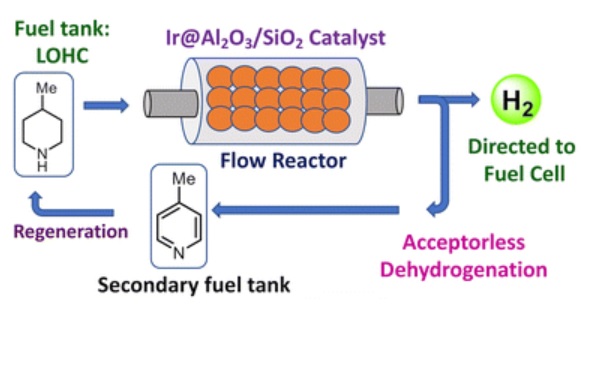
But there is also another option – liquid organic hydrogen carriers (LOHCs). These are organic molecules that can bind a lot of hydrogen and reversibly release it. They can be stored as a liquid, like regular fuel, at ambient temperatures and pressures. However – they have some technical limitations. Their energy density gets up to only about 25% of gasoline. That’s worse than liquid hydrogen, and about the level of compressed hydrogen gas. But we already have compressed gas hydrogen fuel cells cars, so perhaps LOHCs can replace them. Another limitation is that the hydrogen is liberated from the LOHCs at 350 C, and stored at 150C (or similar, depending on the specific molecule used). This is energy intensive and reduces the energy efficiency of the system. Further, in a car, for example, you would need one tank for the hydrogen-containing LOHC, and then another tank for the hydrogen depleted LOHC (so two 50 gallon tanks). When you refuel, you would need to both drain the spent fuel and replace fresh fuel.
That’s a lot of complex engineering to pack into a car. One of the big advantages of BEVs is that they are mechanically much simpler than gasoline or hydrogen cars. They are solid state, with a battery and motors. They require less labor to build, and require much less maintenance. An LOHC-based car would have to compete with that. But again, there may be a niche where the trade-offs of LOHCs are better than batteries, such as situations in which weight matters more than volume.
So what did the new study add? This is a basic science, proof of concept, study looking at a new catalyst for the release of hydrogen from an LOHC. They used isopropanol and 4-methylpiperidine as the LOHC. The enzyme requires a small amount of iridium, which is a rare metal, but probably not a deal-breaker. It is, according to the study, >99 efficient in stripping hydrogen from the LOHC. This is great, and shows promise. But, it also loses 25% efficiency after 45 hours of use. That is no-so-great.
What we have is an incremental advance in one aspect of the LOHC-as-fuel technology. It does not deal with the main drawbacks of the technology, however. I get that the press release needs to focus on the positive, and the potential of a new technology, but reading deeply into the science and the background of this technology, it’s pretty clear that it’s not ready for prime time. It is difficult to envision a future version of this technology, with the energy density limitations, that is a viable competitor to BEVs. But perhaps it may be the solution for some applications, like trains or large trucks. It may also see a role in industrial applications of hydrogen (not in the transportation sector) where energy density is much less of an issue. There, it has a significant advantage of long term storage at ambient temperatures and pressures.






