May 10 2021
Sequestering Carbon
 The climate and environment are always changing, but this statement is not that meaningful unless you put it into the perspective of timescale. Over very short timescales, a few years, climate is extremely stable. Over very long timescales, millions of years, climate can change dramatically, turning lush rainforests into deserts. Over hundreds of years the climate has been relatively stable, except for natural oscillating cycles. Climate can even be relatively stable over thousands of years, but at this scale we do start to get into the bigger ice-age and glaciation cycles.
The climate and environment are always changing, but this statement is not that meaningful unless you put it into the perspective of timescale. Over very short timescales, a few years, climate is extremely stable. Over very long timescales, millions of years, climate can change dramatically, turning lush rainforests into deserts. Over hundreds of years the climate has been relatively stable, except for natural oscillating cycles. Climate can even be relatively stable over thousands of years, but at this scale we do start to get into the bigger ice-age and glaciation cycles.
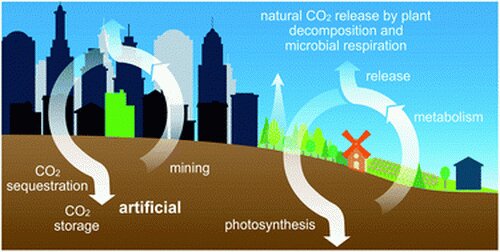
On this backdrop we have the anomalous forcing of global average temperature increases over the last 150 years that cannot be explained as part of any natural cycle. This does correlate with the release into the environment of free carbon dioxide, the carbon component of which had been previously sequestered for millions of years in the form of fossil fuels. Obviously we are in the midst of a big conversation about how to reduce the industrial release of more carbon to limit effects on the environment. While sincere efforts are being made, the pessimistic view is that we simply lack the political will to do what needs to be done, and there are too many economically vested interests in the cheap energy provided by fossil fuel. We will eventually convert to cheaper and cleaner renewable energy sources, because market forces are moving in that direction, but probably not before we burn through our entire “carbon budget”.
The other option for limiting the effects of anthropogenic climate change is to capture and sequester carbon. Trees and other living things are an efficient way to capture carbon, but the sequestration is only temporary. Eventually plant matter decomposes and releases the carbon back into the atmosphere. However, a significant amount of environmental carbon can be stored in plant life, especially trees, and even though this is dynamic it can keep a large amount of carbon from the atmosphere. Right now we are going in the wrong direction, “Over the decade since 2010, the net loss in forests globally was 4.7 million hectares per year.” Reversing this trend can be one of the quickest ways we can mitigate carbon release.
But again, this is only going to get us so far. What we truly need is a way to re-sequester carbon in a more permanent form. Carbon can be a highly useful material, so it should be possible to make useful stuff out of carbon pulled from the atmosphere. We already have the technology to make many carbon-based materials. The real question is scale, cost, and energy efficiency. A recent study gives an example of one type of technology.
The process uses plants to capture the carbon. This makes sense in that plants are a very efficient method of capturing atmospheric carbon and turning it into high energy molecules, such as sugars). The downside is that we are land-limited in our food production and would not want a process that requires a lot of land. However, this process can use plant waste, such as corn stalks, and so could use existing agriculture to source material. I did not see an analysis of how much material this would be, so it remains to be seen how scalable this process truly is.
The sequestration comes from converting the carbon in plant matter into a useful material, in this case silicon carbide (SiC), also known as carborundum. This is an ultrahard material used in ceramics, sandpaper, semiconductors and LEDs. If the plan is to make lots of this material, then it is likely that further uses will be found for it.
But I don’t think this specific technology is going to be the answer. There are two main problems with it, the first is that the process requires heating the plant material with the silicon up to 1600 degrees C. That, of course, is very energy intensive and may even make the entire process counterproductive. The authors try to get around this by saying that eventually the required energy will come from renewable sources, but that does not really counter this limitation. We still need to produce all that energy, and it will be a long time before most of our energy is renewable.
The second problem is that heating the plant material releases most of the captured carbon – only 14% is left.
So yes, this might be an industrially useful way to make a needed material, and might sequester some amount of carbon, but because of the energy requirements and low carbon efficiency, this is not going to be a scalable solution for carbon sequestration.
Right now most carbon capture and sequestration strategies involve temporary storage in plant-matter, or burying the carbon under ground in some form. It would be nice to add to this creating industrially useful and stable materials. SiC is one option, but it’s not a great one.
There are researchers working on other options, like making carbon nanofibers from atmospheric CO2. The challenge with this one is scaling it up. A prototype plant makes 10g per hour – a drop in the bucket.
This is another one of those technologies that requires a suite of characteristics at the same time in order to be really useful. We need a process that uses little energy, produces a stable and useful carbon compound, does not require a lot of land use or limited resources, and can be scaled up in a cost-effective way. Unless the process can be done on a massive scale, it’s not really useful.
We are not there yet, but there are a number of promising technologies.






