Sep 21 2023
Immune Cells to Fight Cancer
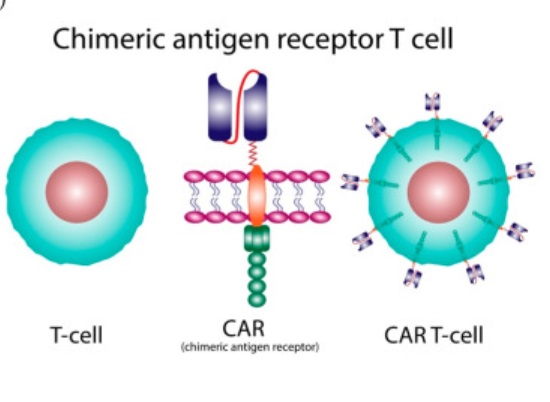 There is a recent medical advance that you may not have heard about unless you are a healthcare professional or encountered it from the patient side – CAR-T cell therapy. A recent study shows the potential for continued incremental advance of this technology, but already it is a powerful treatment.
There is a recent medical advance that you may not have heard about unless you are a healthcare professional or encountered it from the patient side – CAR-T cell therapy. A recent study shows the potential for continued incremental advance of this technology, but already it is a powerful treatment.
Like all technologies, the roots go back pretty far. It was first discovered that immune cells protect against cancer in 1960. In 1986 tumor-infiltrating lymphocytes were used to attack cancer cells. In 1993 the first generation of genetically modified T-cells using the chimeric antigen receptor (CAR) technique was developed. This technique fuses the business end of an antibody to a receptor on a T-cell (hence a chimera) which potentially targets that T-cell against whatever antigen the antibody targets. So you can make antibodies against a protein unique to a specific type of cancer, then fuse part of that antibody to T-cell receptors, put those T-cells back into the patient and they will potentially attack the cancer cells. This first generation CAR-T cells were not effective clinically because they did not survive long enough in the body to work.
In 1998 it was discovered that adding a costimulatory domain (CD28) to the CAR allowed it to persist longer in the body, creating the potential for clinical treatment. In 2002 this technique was used to develop second generation CAR-T cells that were shown for the first time to fight cancer in mice (the first study was of prostate cancer). Then in 2003 CAR-T cells using CD19 instead of CD28 were developed, and found to be more effective. In 2013 the first clinical trial in humans showing efficacy of CAR-T therapy in cancer (leukemia) was published, starting the era of using CAR-T therapy in treating blood cancers. The first CAR-T base treatment was approved by the FDA in 2017.
Over the last 8 years CAR-T therapy has become standard treatment for blood-born cancers (lymphoma and acute lymphoblastic leukemia). T-cells are taken from patients with cancer, they are then genetically modified into CAR-T cells, reproduced to make lots of them, and then given back to the patient to fight their cancer. In the last decade scientific advance has continued, with research targeting other proteins to potentially fight solid tumors. In 2017 scientists starting using CRISPR technology to make their CAR-T cells allowing greater control and improved function.
While these are effective treatments that have improved the duration and quality of life for many patients, the technology is still limited. As many as 60% of CAR-T treated patient will relapse. There can also be serious side effects, most notably cytokine release syndrome – an activation of the immune system that can cause serious side effects. For these reasons there is constant tinkering with the CAR-T protocols. It has only been 8 years since approval, and there are lots of variables to work out, such as coadministering chemotherapy, timing of treatments, and treatment to minimize side effects.
In addition, the CAR-T technology itself is improving, with new versions designed to target solid tumor, to have greater efficacy and fewer side effects. Even incremental improvements at this point should lead to steady improvement in outcomes.
The recent study that triggered my review of the technology is representative of the kinds of research going on – Reductive carboxylation epigenetically instructs T cell differentiation. That’s a boring technical title, so let’s break it down. The problem the researchers are interested in is the fact that CAR-T cells do not last very long in the body. This was the original problem with the technology, and has been improved to the point of clinically effectiveness, but still remains a limitation. What happens is that the T-cells reproduce rapidly, which causes them to become “exhausted” – which is a technical term meaning that they are no longer able to reproduce. If we could find ways to delay exhaustion, then CAR-T treatments would be more effective and perhaps reduce relapse.
Rapidly reproducing T-cells and rapidly reproducing cancer cells have something in common – they rely on an alternative metabolic pathway to compensate for not having enough oxygen to meet their energy needs. Specifically, they use reductive carboxylation to get energy from the amino acid glutamine. This pathway also modifies the proteins (histones) that wrap up DNA. These proteins will unwind regions of DNA to make those genes accessible for transcription (allow them to be active), and wrap up DNA for genes that are not needed. When the reductive carboxylation pathway is active, histones are modified in such a way that genes for active inflammation and reproduction are active, while genes for longevity are not active. This causes the T-cells to burn out and become exhausted.
The researchers found that they can inhibit the reductive carboxylation enzyme without affecting the function of the CAR-T cells. They continue to work just fine. But they slow their reproduction and last a lot longer. This shifts the cells out of the active phases where they burn themselves out to a more longevity phase. This is basic science, but it can potentially be “translatable” – meaning that it can translate into clinical effectiveness. Ideally this will allow CAR-T cells to function much longer and increase their effectiveness.
This is just one incremental basic science advance. It may not even pan out clinically. However, it shows the level of research that is being done, and the tremendous potential for genetic engineering of CAR-T cells to tweak their function. We are at the beginning of the CAR-T cell era of medical intervention. More broadly, we are at the beginning of the era of using genetic engineering to modify cells for therapeutic potential, with CAR-T cells just being one application. The potential power of this approach is incredible.






