Jan 16 2024
Betavoltaic Batteries
 In 1964 Isaac Asimov, asked to imagine the world 50 years in the future, wrote:
In 1964 Isaac Asimov, asked to imagine the world 50 years in the future, wrote:
“The appliances of 2014 will have no electric cords, of course, for they will be powered by long- lived batteries running on radioisotopes. The isotopes will not be expensive for they will be by- products of the fission-power plants which, by 2014, will be supplying well over half the power needs of humanity.”
Today nuclear fission provides about 10% of the world’s electricity. Asimov can be forgiven for being off by such a large amount. He, as a science fiction futurist, was thinking more about the technology itself. Technology is easier to predict than things like public acceptance, irrational fear of anything nuclear, or even economics (which even economists have a hard time predicting).
But he was completely off about the notion that nuclear batteries would be running most everyday appliances and electronics. This now seems like a quaint retro-futuristic vision, something out of the Fallout franchise. Here the obstacle to widespread adoption of nuclear batteries has been primarily technological (issues of economics and public acceptance have not even come into play yet). Might Asimov’s vision still come true, just decades later than he thought? It’s theoretically possible, but there is still a major limitation that for now appears to be a deal-killer – the power output is still extremely low.
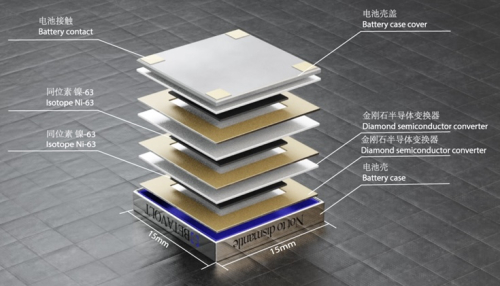
Nuclear batteries that run through thermoelectric energy production have been in use for decades by the aerospace industry. These work by converting the heat generated by the decay of nuclear isotopes into electricity. Their main advantage is that they can last a long time, so they are ideal for putting on deep space probes. These batteries are heavy and operate at high temperatures – not suitable for powering your vacuum cleaner. There are also non-thermal nuclear batteries, which do not depend on a heat gradient to generate electricity. There are different types depending on the decay particle and the mechanism for converting it into electricity. These can be small cool devices, and can function safely for commercial. In fact, for a while nuclear powered pacemakers were in common use, until lithium-ion batteries became powerful enough to replace them.
One type of non-thermal nuclear battery is betavoltaic, which is widely seen as the most likely to achieve widespread commercial use. These convert beta particles, which are the source of energy –
“…energy is converted to electricity when the beta particles inter-act with a semiconductor p–n junction to create electron–hole pairs that are drawn off as current.”
Beta particles are essentially either high energy electrons or positrons emitted during certain types of radioactive decay. They are pretty safe, as radiation goes, and are most dangerous when inhaled. From outside the skin they are less dangerous, but high exposure can cause burns. The small amounts released within a battery are unlikely to be dangerous, and the whole idea is that they are captured and converted into electricity, not radiated away from the device. A betavoltaic device is often referred to as a “battery” but are not charged or recharged with energy. When made they have a finite amount of energy that they release over time – but that time can be years or even decades.
Imagine having a betavoltaic power source in your smartphone. This “battery” never has to be charged and can last for 20-30 years. In such a scenario you might have one such battery that you transfer to subsequent phones. Such an energy source would also be ideal for medical uses, for remote applications, as backup power, and for everyday use. If they were cheap enough, I could imagine such batteries being ubiquitous in everyday electronics. Imagine if most devices were self-powered. How close are we to this future?
I wish I could say that we are close or that such a vision is inevitable, but there is a major limiting factor to betavoltaics – they have low power output. This is suitable for some applications, but not most. A recent announcement by a Chinese company, Betavolt, reminded me of this challenge. Their press release does read like some grade A propaganda, but I tried to read between the lines.
Their battery uses nickel-63 as a power source, which decays safely into copper. The design incorporates a crystal diamond semiconductor, which is not new (nuclear diamond batteries have been in the news for years). In a device as small as a coin they can generate 100 microwatts (at 3 volts) for “50 years”. In reality the nickel-63 has a half-life of 100 years. That is a more precise way to describe its lifespan. In 100 years it will be generating half the energy it did when manufactured. So saying it has a functional life of 50 years is not unreasonable.
The problem is the 100 microwatts. A typical smart phone requires 3-5 watts of power. So the betavolt battery produces only 1/30 thousandth the energy necessary to run your smart phone. That’s four orders of magnitude. And yet, Betavolt claims they will produce a version of their battery that can produce 1 watt of power by 2025. Farther down in the article it says they plan-
“to continue to study the use of strontium 90, plethium 147 and deuterium and other isotopes to develop atomic energy batteries with higher power and a service life of 2 to 30 years.”
I suspect these two things are related. What I mean is that when it comes to powering a device with nuclear decay, the half-life is directly related to power output. If the radioisotope decays at half the rate, then it produces half the energy (given a fixed mass). There are three variables that could affect power output. One is the starting mass of the isotope that is producing the beta particles. The second is the half life of that substance. And the third is the efficiency of conversion to electricity. I doubt there are four orders of magnitude to be gained in efficiency.
From what I can find betavoltaics are getting to about the 5% efficiency range. So maybe there is one order of magnitude to gain here, if we could design a device that is 50% efficient (which seems like a massive gain). Where are the other three orders of magnitude coming from? If you use an isotope with a much shorter half-life, say 1 year instead of 100 years, there are two orders of magnitude. I just don’t see where the other one is coming from. You would need 10 such batteries to run your smart phone, and even then, in one year you are operating at half power.
Also, nuclear batteries have constant energy output. You do not draw power from them as needed, like with a lithium-ion battery. They just produce electricity at a constant (and slowly decreasing) rate. Perhaps, then, such a battery could be paired with a lithium-ion battery (or other traditional battery). The nuclear battery slowly charges the traditional battery, which operates the devices. This way the nuclear battery does not have to power the device, and can produce much less power than needed. If you use your device 10% of the time, the nuclear battery can keep it charged. Even if the nuclear battery does not produce all the energy the device needs, you would be able to go much longer between charges, and you will never be dead in the water. You could always wait and build up some charge in an emergency or when far away from any power source to recharge. So I can see a roll for betavoltaic batteries, not only in devices that use tiny amounts of power, but in consumer devices as a source of “trickle” charging.
At first this might be gimicky, and we will have to see if it provides a real-world benefit that is worth the expense. But it’s plausible. I can see it being very useful in some situations, and the real variable is how widely adopted such a technology would be.






