Jan 11 2022
The Man with the Pig Heart
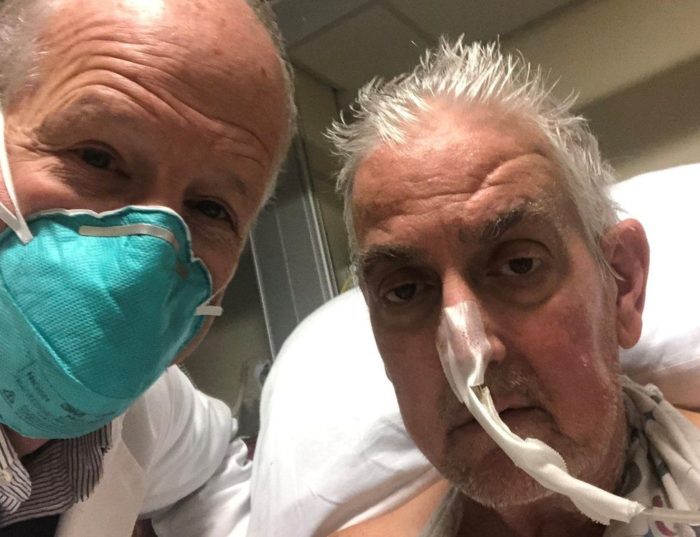 David Bennett, 57, had terminal heart disease. He was bed-ridden and kept alive on a heart machine for the last six weeks, a temporary measure at best. He was deemed too sick for a donor heart transplant, which are in limited supply and given to the patients most likely to benefit from them. Essentially, his options were over and death was imminent and unavoidable. For this reason he was considered a viable candidate for an experimental procedure, and the FDA granted emergency use authorization under its compassionate use guidelines.
David Bennett, 57, had terminal heart disease. He was bed-ridden and kept alive on a heart machine for the last six weeks, a temporary measure at best. He was deemed too sick for a donor heart transplant, which are in limited supply and given to the patients most likely to benefit from them. Essentially, his options were over and death was imminent and unavoidable. For this reason he was considered a viable candidate for an experimental procedure, and the FDA granted emergency use authorization under its compassionate use guidelines.
On January 7th he received a heart transplant from a pig that had been genetically modified to minimize rejection. This is a true milestone – the first successful transplant of a living organ from a non-human donor into a living human (organ xenotransplantation). The reason for the caveats are the fact that pig valves are routinely transplanted into people, but these valves are fixed and therefore not living tissue. Also, you may remember the girl with the baboon heart, Baby Fae, an infant who received a baboon heart in 1984. She lived for 21 days, but this was not considered a viable procedure, which is why it was not repeated. Also, last year a genetically modified pig kidney was transplanted into a human, but they were brain dead at the time.
It remains to be seen how long David Bennett with survive with his new pig heart. Rejection is still a major issue, and he will need to be on powerful immunosuppressant drugs. There is also a reason he was not considered a good candidate for a human heart transplant. But even if the procedure is moderately successful this would represent a true milestone, our entry into the age of routine organ xenotransplantation with genetically modified organs.
The need is obviously great. In the US alone there are 100,000 people on the waiting list for an organ transplant, and ten patients die each day while on the list. An elaborate system is in place to connect donors with recipients in the most efficient way. We will still need this organ donation program for years to come, but the addition of xenografts is a definite game-changer.
The actual surgery was conducted at the University of Maryland Medical Center in Baltimore by Dr. Bartley P. Griffith. Not taking anything away from this accomplishment, but the real achievement here is all the science that led up to the surgery. The donor pig had four genes knocked out, three of which for proteins that would cause rejection, and one to limit the growth of the pig heart so it doesn’t become too big. There were also six human genes inserted into the pig to make it more immunologically compatible. I could not find a report of the specific method used, but it was probably CRISPR (but could have also been Talen or Zinc Fingers).
Immune rejection is the biggest problem with organ donors. Even human to human donation can cause rejection. This occurs because our immune systems are able to distinguish self from non-self by identifying specific proteins on the outside of cells. Anything it detects as non-self it attacks as a foreign invader. To minimize this, donors are immunologically matched to recipients. The closer a donor is genetically to the recipient, the less this immune attack will be.
Now imagine how different another species is from a human recipient. This is why the baboon heart transplant had no chance of success (and why it was so controversial and not repeated). The only chance for such xenografts to work (even with immunosuppression) is if the non-human donors are genetically modified to be more immunologically human. That is precisely what was done with the genetic engineering. Now that we have crossed the line (assuming all goes reasonably well) of viability, the goal will be to constantly tweak the genetic engineering to further minimize any rejection. Ideally researchers will be able to make germline genetic changes so that compatible donors can be bred, without having to be genetically engineered individually. In this way we can literally grow organs for transplantation.
Theoretically, once we deal with the human-pig differences, it would be possible to further tweak the pig genetics to make specific types compatible with different human subpopulations (like blood types). Perhaps we will start with a generically humanized pig, then do further genetic engineering to be more compatible with a specific intended recipient. The closer we can get the donor to the recipient genetically, the better. It may even be possible to target one individual recipient. The pigs do take time to grow to a sufficient size for the donation, but often patients are on a waiting list for years, time enough to grow a pig organ. This procedure can work theoretically for any organ that can be transplanted, not just hearts.
At first xenotransplants will be used, as in this case, for patients who are otherwise terminal and not a candidate for a human donor. As the technology improves and becomes more established, they can be used as an alternative to waiting on the human donor list. This in turn will reduce demand for human donors, taking a lot of pressure off the entire system. Eventually, assuming the technology continues to improve, we may have pig donors that are so genetically tweaked they are even superior to human donors (unless you have an identical twin or very close living relative). In this case the pig donors will essentially replace human donors.
The ultimate expression of this technology is the ability to grow pig (or other animal) donors that are immunologically genetically identical to the intended recipient. This would not only replace human donors, but would eliminate the need for anti-rejection drugs. I can’t think of any theoretical obstacle to this ultimate outcome, so it does seem like more of a matter of when than if. It’s always hard to know about unanticipated problems until we actually do something, but the science here looks solid.
This does feel like a “first test-tube baby” kind of moment, our first steps into a new technology that will revolutionize organ transplantation.






