Aug 22 2017
Bacterial Solar Cells
 Plants make energy from light – a neat trick we would like to harness for our own use. Specifically, plants can turn sunlight, carbon dioxide and water into sugars, which are molecules that contain a lot of energy. Sugars can be turned into ethanol, which can be burned as a fuel. This is the essence of biofuels. The advantage of this process is that it is carbon neutral. When you burn the biofuel you are returning CO2 to the atmosphere that was previously removed when the biofuel was made. You are not releasing previously sequestered CO2 into the carbon cycle.
Plants make energy from light – a neat trick we would like to harness for our own use. Specifically, plants can turn sunlight, carbon dioxide and water into sugars, which are molecules that contain a lot of energy. Sugars can be turned into ethanol, which can be burned as a fuel. This is the essence of biofuels. The advantage of this process is that it is carbon neutral. When you burn the biofuel you are returning CO2 to the atmosphere that was previously removed when the biofuel was made. You are not releasing previously sequestered CO2 into the carbon cycle.
The problem with biofuels is that the process is inefficient. The overall process is about 1% efficient in terms of how much of the sunlight falling on a field of crops used for biofuels ends up as usable fuel. Photosynthesis itself is only about 3-6% efficient.
While there may be a niche for biofuels in the future, I doubt they will make a sizable dent in our energy needs. The ultimate problem is land use. We are pretty much already using all available land to grow food to eat, and a growing population will make this more challenging. So we simply don’t have land to dedicate to growing crops specifically for biofuels. We can use crop waste and non-food plants, but that will likely not be enough to be a major contributor to our energy infrastructure.
If any kind of biofuel is going to be worth it, we have to get the efficiency way up. Some scientists think that plants are not going to be the solution. They are simply too inefficient at harnessing light energy. If we want a self-replicating organism that can turn atmospheric CO2 and sunlight into a biofuel with high efficiency, we have to turn to bacteria. A recent study demonstrates that this option might be viable.
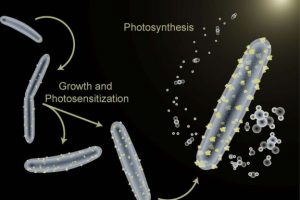
Some bacteria will process toxic metals by excreting them as crystals. Dr. Kelsey Sakimoto from Harvard who was involved with the study explains:
“It’s shamefully simple, we’ve harnessed a natural ability of these bacteria that had never been looked at through this lens. We grow them and we introduce a small amount of cadmium, and naturally they produce cadmium sulphide crystals which then agglomerate on the outsides of their bodies.”
The cadmium sulphide crystals allow the bacteria to harness sunlight and produce acetic acid (vinegar – CH3COOH). The good news is that this process, they claim, is 80% efficient. That is much higher than photosynthesis with plants. You can basically just grow a vat of the bacteria, mix in a little cadmium, expose it to light, and crank out acetic acid with high efficiency.
The next question is – what do you do with the acetic acid? You have gotten the carbon in CO2 into a higher energy molecule, and that’s the tough part that requires energy input. But you can’t put vinegar into your fuel tank. But you can make butanol from acetic acid, and butanol can be burned like gasoline (even in a regular car engine without modification).
This process is an interesting possibility, especially since it does not require much land, only vats of bacteria, and the efficiency is reportedly high. But of course, it remains to be seen if a viable total process leading to a usable biofuel can be scaled up and industrialized, and what the overall efficiency of that mass production would be. This specific pathway may not be the one that is ultimately used (if anything is).
It does show, however, that there is a lot of interesting research in this arena with tantalizing results. As with other energy production and storage technologies, the process would need to have a number of beneficial features simultaneously to be useful. This one has a few at least – cheap and available inputs and reasonable efficiency. But there are still unknowns, like what it will take to scale up the process.
Of course, for any biofuel, or any process to make energy, to have a significant impact on our total energy infrastructure, it needs to be scalable to a massive size. Getting plants, yeast, or bacteria to do interesting things in the lab is a start, but unless the process makes sense at a large scale it won’t lead anywhere.
I still think biofuels are the long shot in this race. I doubt they will achieve anything more than a small niche in energy production. But it is good that there is ongoing research. Living organisms have tremendous potential, if we can piece together a production chain to a final product that works at scale.






