Oct 18 2021
The Source of Elements
 Perhaps the most famous line from Carl Sagan’s Cosmos series is, “We’re made of star stuff”. In this statement Sagan was referring to the fact that most of the elements that make up people (and everything else) were created (through nuclear fusion) inside long dead stars. While this core claims is true, physicists are finding potential supplemental sources of heavy elements, including in some surprising locations.
Perhaps the most famous line from Carl Sagan’s Cosmos series is, “We’re made of star stuff”. In this statement Sagan was referring to the fact that most of the elements that make up people (and everything else) were created (through nuclear fusion) inside long dead stars. While this core claims is true, physicists are finding potential supplemental sources of heavy elements, including in some surprising locations.
Elements are defined entirely by their number of protons, with hydrogen being the lightest element at one proton. The isotope of an element is determined by the number of neutrons, which can vary. The number of electrons are determined by the number of protons. An atom with greater or fewer electrons than protons is an ion, because it carries an electric charge. The next lightest element is helium, at two protons, and then lithium, at three protons. Current theories, observations, and models suggest that these three elements were the only ones created in the Big Bang, in a process known as nucleosynthesis.
It actually took about 1 second after the Big Bang for the universe to cool enough for protons and neutrons to exist, and then for the next three minutes or so all the elemental nuclei formed – about 75% hydrogen, 25% helium, and trace lithium (by mass). It then took 380,000 years for the universe to cool enough for these nuclei to capture electrons. Where, then, did the elements heavier than lithium come from? This is where Sagan’s “star stuff” comes in.
Stars are fueled by nuclear fusion in their cores, the process of combining lighter elements into heavier elements. The more massive the star, the more heat and pressure they can generate in their core, and the heavier the elements they can fuse. Fusion of these elements is exothermic, it produces the energy necessary to sustain the fusion. This lasts until enough of the heavier fusion product builds up in the core to effectively stop fusion. Then the star will collapse further, becoming hotter and denser until it can start fusing the heavier element. This process continues until the star is no longer able to fuse the elements in its core because it is simply not massive enough, there is therefore nothing to stop its collapse and it becomes a white dwarf.
Even for the most massive stars, however, they cannot have sustained fusion beyond iron (element 26). The fusion or fission of iron is endothermic, so there is no way to sustain fusion of a core of iron. Also, for the more massive stars, when they collapse they will have enough energy to produce a supernova – the core collapses so fast and with so much energy it is able to fuse even heavier elements in a giant explosion. These events leave behind stellar remnants that become either a neutron star or a black hole, depending on their mass.
All that I described above is the standard model for where all the elements come from. Hydrogen, helium, and lithium were formed in nucleosynthesis at the Big Bang. Elements up to and including iron (and including more helium and lithium) can be created in the core of stars from stellar fusion. All elements heavier than iron are created during supernova explosions. This is an elegant model that nicely explains the synthesis of all the naturally-occurring elements. But that doesn’t mean that scientists are done there – the question remains, are there any supplemental sources of element formation?
In 2017 astronomers witnessed an event that might be another source of heavier elements – the explosive merger of two neutron stars. This even it terms a kilonova, and was observed through gravitational waves. In 2019 astronomers also analyzed the spectral pattern in the rapidly expanding fireball resulting from the kilonova. This was difficult to analyze because it has no many spectral lines, but they were able to pull out the spectral pattern specific to strontium, a element heavier than iron with an atomic number of 38. This suggests that kilonova can be added to the list of sources of heavy elements. Physicists theorize that neutrino bombardment from the kilonova would decay neutrons into protons and electrons, and the intense energy can fuse these into heavier elements (as in a supernova).
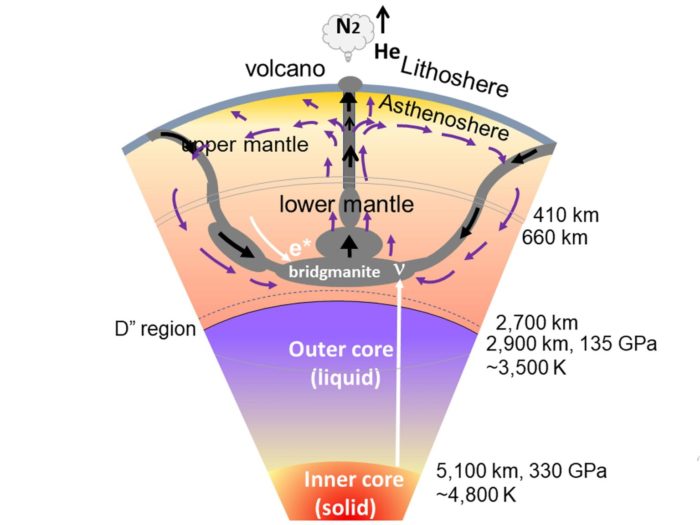
A researcher has now proposed a theoretical source of element formation that at first may seem implausible – the Earth itself. The idea is that the extreme temperatures and pressures within the inner mantle of the Earth may be enough for some fusion to occur. Obviously this would not be a self-sustaining fusion reaction. Rather they hypothesize that the movement of the lithosphere could provide additional energy to the heat and pressure. This process could further be fed by neutrinos from radioactive decay of unstable elements. They write:
Here, we propose that the formation of 25 elements with smaller atomic numbers than iron resulted from an endothermic nuclear transformation of two nuclei confined in the natural compound lattice core of the Earth’s lower mantle at high temperatures and pressures. This process is accompanied by the generation of neutrinos and is influenced by excited electrons generated by stick-sliding during supercontinent evolution, mantle convection triggered by major asteroid collisions, and nuclear fusion in the Earth’s core. Therefore, our study suggests that the Earth itself has been able to create lighter elements by nuclear transmutation.
This process, therefore, would not be a source of elements heavier than iron, which is still limited to powerful stellar events such as supernovae and kilonovae. This could provide a supplemental source of the lighter elements (up to iron). Much further research is needed to figure out if this process is truly possible, and if it actually occurs and to what degree. Now it is just a proposed mechanism, and the paper serves the purpose of pointing in the direction of further research.
Perhaps more interesting is the very notion that scientists continue to look for places in the universe where nuclear transformation can take place resulting in all the naturally occurring elements.






