May 12 2022
Stem Cells for Parkinson’s Disease
 The healing potential of stem cells came to the public consciousness in 2001, when president George Bush banned federal funding for any research involving new embryonic stem cell lines. This sparked a public and professional debate, with proponents touting the amazing potential of stem cells to cure serious diseases, with Alzheimer’s and Parkinson’s frequently mentioned as examples. Critics were worried about the harvesting of human embryos to fuel this research. Bush’s ban was meant to be a compromise, allowing research on existing stem cell lines but banning the creation of new lines. But in practice it was a thorough ban, because existing lines were limited, and also the ban was applied to all federal funding of an entire institution in which any lab was involved in prohibited research. The US likely lost a decade of stem cell research as a result.
The healing potential of stem cells came to the public consciousness in 2001, when president George Bush banned federal funding for any research involving new embryonic stem cell lines. This sparked a public and professional debate, with proponents touting the amazing potential of stem cells to cure serious diseases, with Alzheimer’s and Parkinson’s frequently mentioned as examples. Critics were worried about the harvesting of human embryos to fuel this research. Bush’s ban was meant to be a compromise, allowing research on existing stem cell lines but banning the creation of new lines. But in practice it was a thorough ban, because existing lines were limited, and also the ban was applied to all federal funding of an entire institution in which any lab was involved in prohibited research. The US likely lost a decade of stem cell research as a result.
The Bush ban was reversed by Obama in 2009. Perhaps more importantly, starting in 2007 scientists were publishing research showing how to turn adult cells into pluripotent stem cells. These are not quite as good as embryonic stem cells, which are totipotent (pluripotent can turn into any type of cell in the body, while totipotent can turn into any cell type, including placental cells). But they are close, and also are extremely useful. Over the next decade this research continued, finding new and easier ways to program adult cells into pluripotent stem cells, called “induced” pluripotent stem cells (iPSC). These cells also have a potential advantage – they can be harvested from a person for use in that person, and therefore eliminates the issue of rejection.
Throughout the last two decades, despite the ban, stem cells have been hyped as a potential cure-all, the ability to replace or regenerate tissue and outright cure even the worst degenerative diseases. But reality has not lived up to the hype. There are many technical hurdles to using stem cells in this way – they have to survive and even thrive, take up their desired function, form anatomical structures or connections to other cells, and not form into tumors. That last bit has been a huge barrier to their therapeutic use.
Readers of this blog, however, will recognize a common theme in predicting the future of technological advance – there is a tendency to overestimate short term advance and underestimate long term progress. Two decades ago it seemed stem cells were close to a medical revolution, at least to the public, but experts knew or should have known that the barriers were substantial. Taking 2-4 decades to sort out the technical details was a reasonable estimate at the time, but that sober assessment rarely made it to the public. However, once technical hurdles are sorted, the potential often exceeds even optimistic predictions. We are not there yet with stem cells, and we may still be decades away from this technology being mature. But we are making continuous progress.
On that note, here is a proof-of-concept study that does seem promising, using iPSCs in a rat model to treat Parkinson’s disease (PD). PD is a neurodegenerative disorder where a specific type of neuron (brain cell) dies off. Neurons are a special type of brain cell that makes long connections to other neurons and communicates with them electrochemically, through both electrical and chemical signals. The neurons in question are in a part of the brain called the substantia nigra (specifically the pars compacta), named for its dark or black color. This structure is part of a larger circuit called the extrapyramidal system, which is largely located in the basal ganglia deep in the brain. This circuit is involved with regulating moment-to-moment the smoothness of voluntary muscle control. It’s a feedback circuit carefully regulating the gain of the connection between the conscious desire to move and the resulting movement. Turn it up and people writhe and move continuously, turn it down and they move more and more slowly, even to the point of freezing entirely. Patient with PD move slowly (called bradykinesia), which also makes it difficult to walk, can affect the speech, facial expressiveness, and also cause tremors.
The pars compacta neurons release the neurochemical dopamine, and therefore one of the treatments of PD is to simply give the precursor of dopamine to maximize the amount that surviving neurons can secrete. But as more and more neurons are lost, this strategy becomes less and less effective. Eventually there are no neurons left to buffer the dopamine release, and patients have movement that reflects their blood levels of the drug. They therefore constantly fluctuate from being hyperkinetic to bradykinetic, with brief windows of being just right.
For decades neuroscientist have tried to treat PD by implanting cells in the brain that would directly release dopamine. In 1979 two papers were published showing the potential of transplanting fetal brain tissue into the brains of PD patients. But these cells did not making meaningful connections, they just released dopamine locally. This was essentially a drug-delivery system. Only select patients were candidates, it was difficult invasive surgery, and long term survival of the transplanted cells was a huge issue. So while it remained a choice for some patients, it did not revolutionize the treatment of PD and did not constitute anything close to a “cure”.
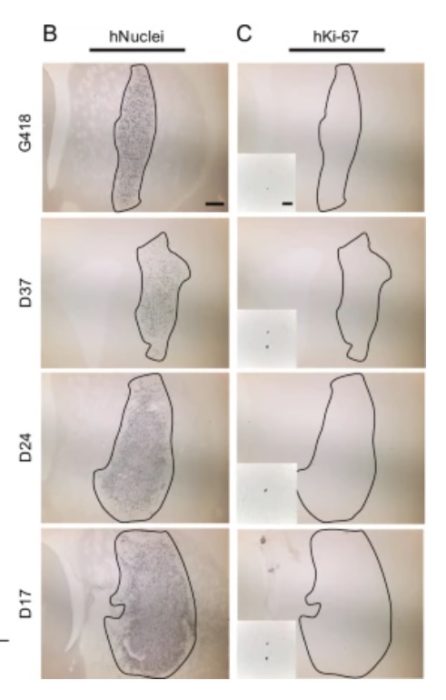
Stem cells are the obvious next step – transplanting programmed neurons that can, theoretically, connect into the circuit and not just release dopamine by regulate the release of dopamine, restoring normal function. This has proven very difficult, but the recent rat study does seem promising. The researchers used adult-derived skin cells and then transformed them into iPSCs. They were specifically trying to figure out the optimal timing of the two-step process. First you have to de-differentiate the cells into stem cells and then you have to program them to become neurons. The timing of each treatment is critical. What they found was that 17 days for the first step was optimal. They used these cells, then differentiated them into neurons and transplanted them into rats with a genetic form of PD. The cells survived, made connections, released dopamine, and reduced the symptoms of PD in the rats. Further, there was a nice dose-response effect. Those rats who receive few cells had a mild benefit, while those with larger implants were virtually cured of any signs of PD. Perhaps most importantly, they did not detect the formation of any tumors as a result.
The next step is human trials with the same type of genetic PD. While these results are exciting, we know now not to get too excited. Until we do the human research we don’t know if this treatment will be viable. First, human brains are much larger than rat brains, this means that the transplanted neurons have to make much longer-distance connections. This is not a trivial factor, and may turn out to be a deal-killer for this approach. If the axons don’t find their targets and form a completed circuit, the treatment will not work as intended. Also a larger volume of cells will be needed, and we need to see if this affects the chance of forming harmful tumors.
In short, while this treatment appears to work in rats, going from rat brains to human brains is not trivial. We can therefore be hopeful, but not celebrate just yet. Clinical trials will likely take years, and if this approach does not work it’s back to the drawing board and it may be another decade before we get to this point. Of course, I really hope this works perfectly. If it does we may be finally getting to the point where we are realizing some of the stem-cell hype from 20 years ago. But don’t be surprised if it takes another 20 years.






