Jan 27 2017
Scientists Create Metallic Hydrogen
 It was just announced that for the first time scientists were able to create a small amount of metallic hydrogen in the lab. This is a significant breakthrough, and is sure to lead to further discovery, although it remains to be seen what specific practical applications may emerge.
It was just announced that for the first time scientists were able to create a small amount of metallic hydrogen in the lab. This is a significant breakthrough, and is sure to lead to further discovery, although it remains to be seen what specific practical applications may emerge.
Hydrogen, as most people know, is a gas at familiar temperatures and pressures. The universe is comprised of about 90% hydrogen. There is very little free hydrogen on the Earth, since it is a very light gas, but there is lots of hydrogen bound up in molecules, such as water.
In 1935, physicists Eugene Wigner and Hillard Bell Huntington hypothesized that under extreme pressure hydrogen atoms may form into a metal – metallic hydrogen. The point at which this happens was named the Wigner-Huntington transition, which explains the title of the recent paper. Metallic hydrogen can further be a liquid, in which the electrons and protons are free flowing, or they can form a crystalline structure and be a solid.
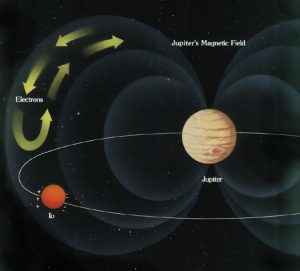
Astronomers infer that the core of Jupiter may be hot liquid hydrogen. We know that Jupiter is made mostly of hydrogen, and we can calculate that the pressure deep in Jupiter’s core must have millions of times the pressure on the surface of the Earth. That is sufficient pressure, according to theory, to compress hydrogen into its metallic form.
We also know from direct measurement that Jupiter has a very strong magnetic field, with 20,000 times the intrinsic strength and a million times the volume of Earth’s puny magnetic field. A strong planetary magnetic field is caused by the rotation of a conducting material. Earth has a core of liquid iron. So, what is at the core of Jupiter that is generating its much more powerful magnetic field? Currently astronomers believe it is a core of liquid metallic hydrogen.
The difficulty in making metallic hydrogen on Earth is creating the extreme pressures. That is what scientists have finally accomplished. They used diamonds, pressure greater than at the center of the Earth (495 GPa), and extremely cold temperature to achieve solid metallic hydrogen.
As stunning as this achievement is, perhaps more important is what comes next. They will slowly release the pressure to see what happens. There are different theories, but as the authors (Isaac Silvera and Ranga Dias) say, we can theorize endlessly, or we could just see what happens. The big question is – will the metallic hydrogen just revert to regular hydrogen, or will it stay in its metallic state?
The best case scenario will be that the solid metallic hydrogen will be like diamonds, which form under high pressure but are a stable solid once formed. Stable solid metallic hydrogen would likely be a superconductor, and (if it could be produced in quantities – big if) could be used for many electrical applications.
The other possibility is that the metallic hydrogen is metastable at room temperature. This means it is in equilibrium but still will evaporate into gaseous hydrogen from the surface. There are two exciting applications being put forward for metastable metallic hydrogen.
The first is as rocket fuel. When metallic hydrogen turns into gaseous hydrogen it will expand greatly, and can be burned with oxygen. The result would be a rocket fuel with four times the energy density as our current best fuel. That does not sound like much, but it could revolutionize space travel. We could get heavier payloads into orbit with fewer stages.
The second possible application is in fusion reactors. Right now there are efforts to make controlled fusion of hydrogen for the sustained production of clean energy. If we could design and build an operational fusion reactor, that would be a massive game-changer for our energy infrastructure. There is some speculation that metallic hydrogen deuterium may aid in achieving fusion at lower pressures than for regular hydrogen.
There are two huge questions at this time that will determine if metallic hydrogen remains a laboratory curiosity or can be harnessed for practical applications: What happens when the pressure is released, and will it be possible to mass produce?






