Feb 12 2019
Kidney Organoid Breakthrough
 We are on the cusp of several technologies that promise to transform medicine – genetic manipulation, brain-machine interface, and stem cell therapies among them. One of the hopes for stem cells is that we will be able to grow from them entire replacement organs. Imagine that you have kidney failure, and face years of dialysis with the hope of finding a kidney donor, so you can exchange that dialysis for a regimen of anti-rejection drugs. You may wait years on the list because we simply don’t have enough organs to go around.
We are on the cusp of several technologies that promise to transform medicine – genetic manipulation, brain-machine interface, and stem cell therapies among them. One of the hopes for stem cells is that we will be able to grow from them entire replacement organs. Imagine that you have kidney failure, and face years of dialysis with the hope of finding a kidney donor, so you can exchange that dialysis for a regimen of anti-rejection drugs. You may wait years on the list because we simply don’t have enough organs to go around.
Now imagine that even before your kidneys completely fail doctor take a skin or mucous membrane sample from you, and then over the next few month they grow a new kidney from your own cells. The kidney is your own tissue, and so there is no rejection at all. Eventually the new kidney is surgically implanted and you are good to go.
It doesn’t take much imagination to see how awesome this could be. If we could grow new hearts, lungs, livers, pancreases or kidneys from one’s own tissue that would transform medicine. The threshold for doing transplants could go way down, because we no longer need donors, we can grow them. The risk would go down because there is no longer the possibility of rejection and therefore the need for powerful anti-rejection drugs, so we could do transplants in more situations. We wouldn’t have to wait for organs to completely fail. Also, if you have cancer we don’t have to try to preserve as much of the organ as possible – just take the whole thing out, making sure you get all the cancer, and replace it with a new one.
This would be a truly transformative medical advance that would bring us into a new age of medicine. I don’t think we are close (meaning <10 years) to such applications, but we are close enough to say that researchers are working on it, and to see a path to get there. It’s enticing.
The problem is that organs are complicated. They are not just blobs of tissue – they have structure. They are the result of a complex system of self-organization during development that has evolved over hundreds of millions of years. We are now trying not only to control stem cells, but reverse engineer the process of turning cells into tissue and tissue into organs.
One approach is to use a scaffold. You can, for example, take a donated heart, strip it of all the cells leaving behind the fibrous scaffold, and then repopulate that frame with new stem cells derived from the intended recipient. Some advance is being made with this approach, but the massive downside is that it still needs a donated organ. I don’t know if this is the ultimate path we will take, but it may yield some important technology that will find some use.
Another approach is to genetically engineer animals, like pigs, so that their tissue is compatible with humans and will trigger a minimal immune tissue-rejection response. I think we are closest to realizing this approach – it’s mainly a matter of finding the right genetic alterations. Theoretically we could alter animals so that they not only have a human immune profile, but match with subtypes (like blood type), or even with a specific intended recipient. If you know that a heart transplant is in your future, a pig could be engineered with your specific immune type to grow a heart for you. Some people have ethical issues with this approach, but hey, if we’ll sacrifice a pig for bacon, we can sacrifice a pig to save a human life.
The other approach is to grow organs from scratch. One way to do this is to implant the incipient organ (sometimes called an organoid) into an animal (so, in vivo), in order to create the biological environment that will induce development with the proper structure. Growing organs in vitro – outside of an animal host – is even more challenging. We need to recreate all the environmental cues used to induce cells to form the necessary structures.
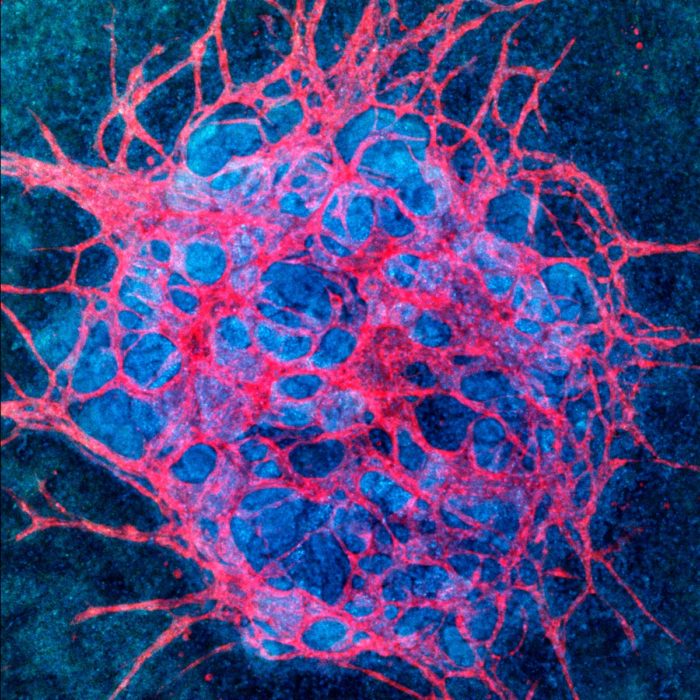
This is the type of advance recently made by researchers at the Wyss Institute for Biologically Inspired Engineering at Harvard University. They are trying to grow kidney organoids in vitro. Their breakthrough is the realization that fluid flow through the kidney organoid is necessary to induce the development of robust vascular supply. Existing kidney organoids have the basic functional unit of a kidney, the nephron. Organoids contain many organized nephrons, but they lack enough blood supply to function as a kidney.
The corresponding author, Ryuji Morizane, said:
“For the first time, our study demonstrates that by exposing growing organoids to fluid flow, a mechanical cue known to play an important role for tissue development in the body, we can greatly enhance their vascularization and maturation in vitro.”
When you think about it, developmental biology is one of the most amazing things in the universe. It is an astoundingly complex dance of self-organization – cells know where to go and how to differentiate based upon their local chemical and physical environment, plus their interaction with other tissues. The body maps to itself and to its own function. We understand a lot of what’s going on, but also that there is much we have not yet figured out. Being able to reproduce the proper environment for self-organization based upon our basic knowledge is going to be challenging. This is one significant step forward, but more will be necessary.
Growing entire functional organs in vitro is the holy grail – no animals or donors necessary, just a small tissue scraping and some time – replacement parts made to order.
There is no theoretical reason why we cannot accomplish this goal. It is just a matter of working out the technology.






