Jan
12
2021
 Would you be willing to pay $35 for a sticker you put on the back of your phone? What if it had “magical” properties that protect you from something that is not harmful in the first place? That is the idea (it seems to me) behind the SmartDot product, made in the UK. On Amazon they claim: “smartDOT Radiation Protection is a low powered magnet programmed with an intelligent combination of natural harmonizing frequencies which reduces harmful EMF radiation emitted by your wireless devices and alleviate symptoms of electro-stress.”
Would you be willing to pay $35 for a sticker you put on the back of your phone? What if it had “magical” properties that protect you from something that is not harmful in the first place? That is the idea (it seems to me) behind the SmartDot product, made in the UK. On Amazon they claim: “smartDOT Radiation Protection is a low powered magnet programmed with an intelligent combination of natural harmonizing frequencies which reduces harmful EMF radiation emitted by your wireless devices and alleviate symptoms of electro-stress.”
This is now boilerplate EMF pseudoscience. What are “harmonizing frequencies”? Nothing – this does not even exist as a concept in science. It’s just nice-sounding jargon for the scientifically illiterate. Also, EMF from smartphones are not dangerous and do not cause any known health issues. Further still there is no such thing as “electro-stress”.
One thing I wanted to point out is what happened when the BBC investigated these stickers. They report:
“But University of Surrey tests for BBC News found no evidence of any effect.”
Total lack of surprise there. The stickers were just stickers, with no energy, no field, and no apparent effect that could be detected. The company responded in a typical way – to make their claims essentially unfalsifiable, or at least as difficult as possible to falsify.
“The Devon-based company told BBC News the stickers were programmed with “scalar energy”, which the scientists’ equipment would be unable to detect.”
Continue Reading »
Dec
17
2020
 As the first crop of SARS-CoV-2 vaccines are being rolled out, we can start to see the light at the end of this pandemic tunnel. Still, in the US alone there will probably be another 200,000 deaths before we reach herd immunity. Further, as fast as this vaccine development and deployment was, it’s not clear how much faster it will bring an end to the pandemic. We will likely be 18 months into the pandemic before we have significant vaccine uptake, and that may not be far off from how long the pandemic was going to last in the first place. There is no doubt is will save lives and hasten the end, but still – only after we lost about half a million people to the virus.
As the first crop of SARS-CoV-2 vaccines are being rolled out, we can start to see the light at the end of this pandemic tunnel. Still, in the US alone there will probably be another 200,000 deaths before we reach herd immunity. Further, as fast as this vaccine development and deployment was, it’s not clear how much faster it will bring an end to the pandemic. We will likely be 18 months into the pandemic before we have significant vaccine uptake, and that may not be far off from how long the pandemic was going to last in the first place. There is no doubt is will save lives and hasten the end, but still – only after we lost about half a million people to the virus.
The question for the future, therefore, is this – is it possible to develop a vaccine even more quickly? What if we could get a vaccine out, soup to nuts, in just a couple of months. We could have ended the pandemic before it really began. The first shots could have been going out in March, protecting front-line workers and the most vulnerable. This would have dramatically slowed the spread while the general population got the vaccine. By now we would be at the tail end of distribution, not the beginning. The COVID pandemic which disrupted the world would have been more like a mild flu season.
Is this feasible? The answer is a definite yes. In fact, as is now being widely reported, it took Moderna just two days to develop their vaccine. They had the vaccine – in January. This is because Moderna has spent the last 10 years developing the mRNA vaccine technology that they used in their COVID vaccine. This, in fact, is the huge advantage of mRNA vaccines, and partly why this technology was being developed. It worked as designed. As soon as the Chinese government released the genetic sequence of the SARS-CoV-2 virus, that was all Moderna needed. They identified the sequence for the spike protein, plugged that into their vaccine platform, and voila – an mRNA vaccine against the SARS-CoV-2 virus. Actually, I’m sure it was more complex than that, but whatever the technical details, it took only two days. And the core idea here is valid – they really only needed the genetic sequence of the organism, which itself now can be done very quickly.
Continue Reading »
Dec
14
2020
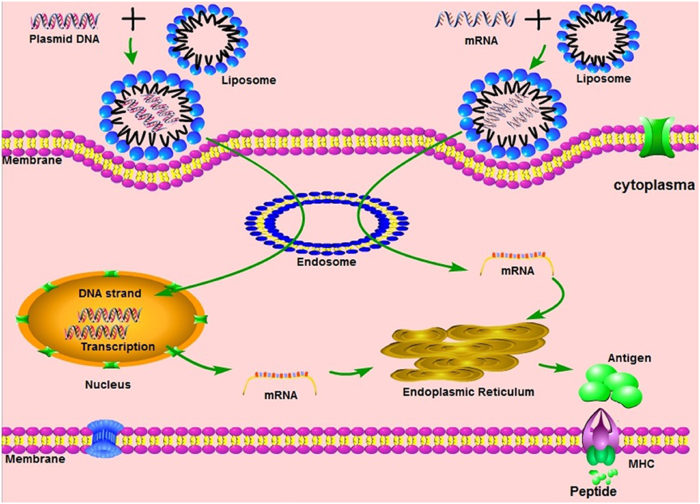 This week should see the first people in the US to actually receive an approved (at least EUA) vaccine to prevent SARS-CoV-2. There are three vaccines currently ready to go in the West, the Pfizer vaccine which received it’s EUA in the US on Friday and was already approved in the UK, Moderna which should get approval this week, and the Astra Zeneca vaccine which should not be too far behind. The (arguably) biggest challenge has been met – a massive scientific effort to develop vaccines in record time. This has been a collaboration between government and industry, and shows what we can accomplish with sufficient motivation (which translates into both money and easing red tape).
This week should see the first people in the US to actually receive an approved (at least EUA) vaccine to prevent SARS-CoV-2. There are three vaccines currently ready to go in the West, the Pfizer vaccine which received it’s EUA in the US on Friday and was already approved in the UK, Moderna which should get approval this week, and the Astra Zeneca vaccine which should not be too far behind. The (arguably) biggest challenge has been met – a massive scientific effort to develop vaccines in record time. This has been a collaboration between government and industry, and shows what we can accomplish with sufficient motivation (which translates into both money and easing red tape).
Now we have three further challenges in front of us. The companies need to mass produce their vaccines. This is happening with about 100 million doses ready to ship. We should have another 2 billion Pfizer doses by the end of 2021, and 1.5 billion Moderna doses. We also need to distribute the doses. This is happening through collaboration among FedEx, UPS, and the military who will get the doses to hospitals and physicians, who can then administer and track the doses. So far, so good.
The final hurdle, however, may prove the stickiest – we need people to accept the vaccine. In a December 9th survey by the AP-NORC, only 47% of Americans said they would get the vaccine, with 26% saying they would not, and 27% saying they are not sure. These and similar results have caused some to comment that the disinformation virus may prove deadlier than the COVID virus. We have a vaccine that can protect people from a deadly pandemic – this is a no-brainer. Resistance is partly due to a dedicated anti-vaccine movement that appears immune only to logic and evidence. We can only marginalize them. But these numbers go beyond the hard-core anti-vaxxers. People also fear what they don’t know, and these are the first mRNA vaccines to hit the market. So let’s review what these are, and the safety data.
Continue Reading »
Nov
30
2020
 I have discussed often before how advances in artificial intelligence (AI) are already transforming our world, but are likely to do so much more in the future (even near term). I am interested in one particular application that I think does not get enough attention – using AI to support clinical decision-making. So I was happy to read that one such project will share in a grant from the UK government.
I have discussed often before how advances in artificial intelligence (AI) are already transforming our world, but are likely to do so much more in the future (even near term). I am interested in one particular application that I think does not get enough attention – using AI to support clinical decision-making. So I was happy to read that one such project will share in a grant from the UK government.
The grant of £20m will be shared among 15 UK universities working on various AI projects, but one of those projects is developing an AI doctor’s assistant. They called this the Turing Fellowship, after Alan Turing, who was one of the pioneers of machine intelligence. As the BBC reports:
The doctor’s assistant, or clinical colleague, is a project being led by Professor Aldo Faisal, of Imperial College London. It would be able to recommend medical interventions such as prescribing drugs or changing doses in a way that is understandable to decision makers, such as doctors.
This could help them make the best final decision on a course of action for a patient. This technology will use “reinforcement learning”, a form of machine learning that trains AI to make decisions.
This is great to hear, and should be among the highest priority in terms of developing such AI applications. In fact, it’s a bit disappointing that similar systems are not already in widespread use. There are several types of machine learning. At its core, machine learning involves looking for patterns in large sets of data. If the computer algorithm is being told what to look for, then that is supervised learning. If not, then it is unsupervised. If it’s using lots of trial and error, that is reinforcement learning. And if it is using deep neural networks, then it is also deep learning. In this case they are focusing on reinforcement learning, so the AI will make decisions, be given feedback, and then iterate its decision-making algorithm with each piece of data.
Continue Reading »
Nov
24
2020
 Two weeks ago Pfizer and German company BioNTech announced preliminary analysis of their phase 3 trial of an mRNA-based vaccine for SARS-CoV-2 showing it is 90% effective (later updated to 95%). One week ago Moderna announced that they too had promising results from their phase 3 trial, also an mRNA vaccine showing 95% efficacy. This week AstraZeneca announced they have developed a COVID vaccine as well (in partnership with Oxford University).
Two weeks ago Pfizer and German company BioNTech announced preliminary analysis of their phase 3 trial of an mRNA-based vaccine for SARS-CoV-2 showing it is 90% effective (later updated to 95%). One week ago Moderna announced that they too had promising results from their phase 3 trial, also an mRNA vaccine showing 95% efficacy. This week AstraZeneca announced they have developed a COVID vaccine as well (in partnership with Oxford University).
I have not discussed the AstraZeneca vaccine before, so here are the basic facts: “It uses a replication-deficient chimpanzee viral vector based on a weakened version of a common cold virus (adenovirus) that causes infections in chimpanzees and contains the genetic material of the SARS-CoV-2 virus spike protein.” In the study, overall there was a 70% efficacy in reducing symptomatic cases of COVID-19. However, in a subgroup analysis, those receiving a half dose followed by a full dose (instead of two full doses) had 90% efficacy, which is closer to the two mRNA vaccines. It is too early to say if this difference is real, and it does not make biological sense, so the company plans to expand the number of subjects getting this dosing regimen to see if the higher efficacy holds up. One advantage to this vaccine is that it can be stored in a regular refrigerator for up to 6 months, while the mRNA vaccines have to be frozen for transport (the Pfizer vaccine at -70 degrees C, the Moderna vaccine at -20).
All three vaccines have a good safety profile so far, but it always takes time to monitor for side effects so this is an ongoing assessment. Pfizer and Moderna say they can produce over a billion doses by the end of 2021, and AstraZeneca says they can produce 3 billion doses. That is enough for about a third of the world’s population. Of course, there are many other vaccines being developed around the world so these won’t be the only three.
Continue Reading »
Oct
22
2020
 Over the course of the pandemic the death rate in people diagnosed with COVID-19 (the case-fatality rate) has declined. Unpacking all the reasons this may be the case can help us better understand and fight this disease. A few recent studies shed some light on this question. While there might be some encouraging news here, it highlights that this is still a “novel” virus and we have a lot to learn about the illness it causes.
Over the course of the pandemic the death rate in people diagnosed with COVID-19 (the case-fatality rate) has declined. Unpacking all the reasons this may be the case can help us better understand and fight this disease. A few recent studies shed some light on this question. While there might be some encouraging news here, it highlights that this is still a “novel” virus and we have a lot to learn about the illness it causes.
One recent study looking at the case fatality rate in the New York region from March to August found that the death rate for those admitted to the hospital dropped from 27% to 3%. They also found many possible reasons for this dramatic decrease. One is the fact that in March New York hospitals were overwhelmed with COVID cases. They did not have enough ICU beds or ventilators, and doctors were crushed beneath the initial wave of cases of a disease they had no experience with. So simply “flattening the curve” and reducing pressure on hospitals is one important factor.
The most encouraging reason for the decline is the steep learning curve of knowing how to treat those who are seriously ill with COVID. Doctors have learned through direct experience how to better manage COVID patients, and many interventions became standard practice between March and August. For example, it is better to rest patients on their stomach than their back, and it is better to delay ventilation as long as possible. The discovery that steroids can reduce the risk of cytokine storm was perhaps a significant improvement. Some patients now get convalescent plasma, something that obviously could not have happened early on. Remdesevir was given emergency use authorization, but a recent study by the WHO found no survival benefit from this drug (or from hydroxychloroquine, a combination of the anti-HIV drugs lopinavir and ritonavir; and interferon).
While we still do not have a cure for COVID-19 or a proven effective anti-viral, management has significantly improved and this has definitely contributed to survival. However – this is not the only effect, and may not even be the major effect.
Continue Reading »
Oct
13
2020
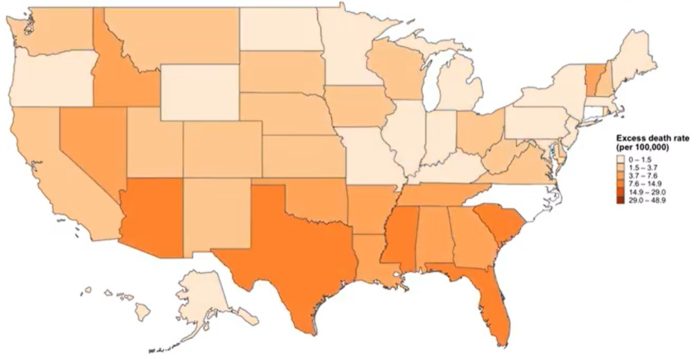 How many people have died in the US so far from the COVID-19 pandemic? It depends on how you count the numbers. The official count of US COVID-19 deaths is 214,000. This number is often reported as “at least” this amount, because this is a compilation of all deaths where COVID-19 was officially listed as a cause of death. Experts recognize that this is likely to be a gross underestimation, because people may die from the disease at home without ever being diagnosed.
How many people have died in the US so far from the COVID-19 pandemic? It depends on how you count the numbers. The official count of US COVID-19 deaths is 214,000. This number is often reported as “at least” this amount, because this is a compilation of all deaths where COVID-19 was officially listed as a cause of death. Experts recognize that this is likely to be a gross underestimation, because people may die from the disease at home without ever being diagnosed.
In any such system, regardless of how careful you are, there are going to be false positives and false negatives. When it comes to the cause of death there are very specific coding guidelines. COVID-19 must have directly lead to the death of the individual. Laboratory confirmation is strongly encouraged, but doctors may code COVID-19 as a probable cause of, in their clinical judgement, the patient had COVID-19 and it fits the epidemiology, even if they did not get a test. When COVID-19 is severe enough to kill, it is a fairly recognizable clinical condition. This does open the door to other fatal viral respiratory infections to be coded as COVID, but these instances are likely to be rare.
States report their data differently. Some only report confirmed cases. Some report confirmed and probable. Some states get their numbers from death certificates, while others count deaths among diagnosed cases of COVID-19. Taking all of this into consideration, COVID-19 deaths are likely to be underestimated in the aggregate rather than overestimated. Some critics argue that allowing “probable” cases overestimates the total deaths from COVID, but if you look at the data state-by-state you will see that probable cases are small in number compared to confirmed. In Arizona, for example, probable cases are only about 5% of the total deaths reports, the vast majority of which are confirmed. So even in the very unlikely scenario that all probable cases are false positives, that only gives a 5% variance (and keep in mind, many states don’t report probable cases at all).
Continue Reading »
Sep
29
2020
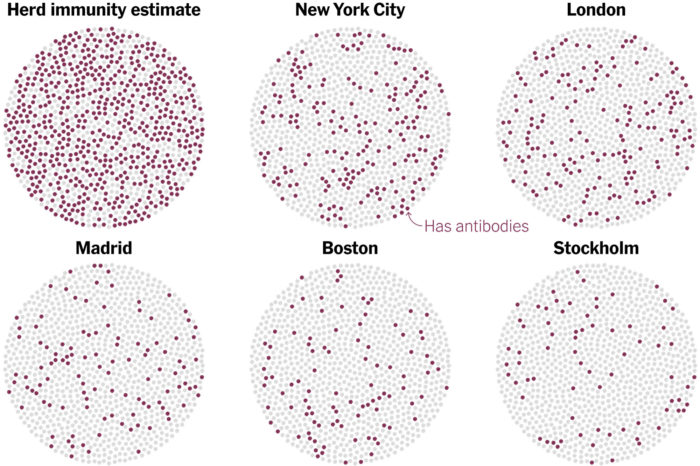 One question weighing on the minds of many people today is – when will this all end? And by “this” (well, one of the “thises”) I mean the pandemic. Experts have been saying all along that we need to buckle up and get read for a long ride on the pandemic express. This is a marathon, and we need to be psychologically prepared for what we are doing now being the new normal for a long time. The big question is – what will it take to end the pandemic?
One question weighing on the minds of many people today is – when will this all end? And by “this” (well, one of the “thises”) I mean the pandemic. Experts have been saying all along that we need to buckle up and get read for a long ride on the pandemic express. This is a marathon, and we need to be psychologically prepared for what we are doing now being the new normal for a long time. The big question is – what will it take to end the pandemic?
Many people are pinning their hopes on a vaccine (or several). This is probably our best chance, and the world-wide effort to quickly develop possible vaccines against SARS-CoV-2 has been impressive. There are currently 11 vaccines in late stage Phase 3 clinical trials. There are also 5 vaccines approved for limited early use. No vaccines are yet approved for general use. If all goes well we might expect one or more vaccines to have general approval by the end of the year, which means wide distribution by the end of 2021. That is, if all goes well. This is still new, and we are fast-tracking this vaccine. This is not a bad thing and does not necessarily mean we are rushing it, but it means we won’t know until we know. Scientists need to confirm how much immunity any particular vaccine produces, and how long it lasts. We also need to track them seriously for side effects.
Early on there was much speculation about the pandemic just burning itself out, or being seasonal and so going away in the summer. Neither of these things happened. In fact, the pandemic is giving the virus lots of opportunity to mutate, and a new more contagious strain of the virus has been dominating since July. Pandemics do eventually end, but that’s not the same as them going away. Some viruses just become endemic in the world population, and they come and go over time. We now, for example, just live with the flu, and with HIV. So perhaps COVID will just be one more chronic illness plaguing humanity that we have to deal with.
But what about herd immunity? The point of an aggressive vaccine program is to create herd immunity – giving so many people resistance that the virus has difficulty finding susceptible hosts and cannot easily spread. The percent of the population with immunity necessary for this to happen depends on how contagious the infectious agent is, and ranges from about 50-90%. We don’t know yet where COVID-19 falls, but this is a contagious virus so will probably be closer to 90%. One question is, how much immunity is the pandemic itself causing, and will we naturally get to herd immunity, even without a vaccine? The results of a new study suggest the answer is no.
Continue Reading »
Sep
24
2020
 Drugs are regulated in most countries for a reason. They can have powerful effects on the body, can be particularly risky or are incompatible with certain diseases, and can interact with other drugs. Dosages also need to be determined and monitored. So most countries have concluded that prescriptions of powerful drugs should be monitored by physicians who have expertise in their effects and interactions. Some drugs are deemed safe enough that they can be taken over the counter without a prescription, usually in restricted doses, but most require a prescription. Further, before going on the market drug manufacturers have to thoroughly study a drug’s pharmacological activity and determine that it is safe and effective for the conditions claimed. There is a balance here of risk vs benefit – making useful drugs available while taking precautions to minimize the risk of negative outcomes.
Drugs are regulated in most countries for a reason. They can have powerful effects on the body, can be particularly risky or are incompatible with certain diseases, and can interact with other drugs. Dosages also need to be determined and monitored. So most countries have concluded that prescriptions of powerful drugs should be monitored by physicians who have expertise in their effects and interactions. Some drugs are deemed safe enough that they can be taken over the counter without a prescription, usually in restricted doses, but most require a prescription. Further, before going on the market drug manufacturers have to thoroughly study a drug’s pharmacological activity and determine that it is safe and effective for the conditions claimed. There is a balance here of risk vs benefit – making useful drugs available while taking precautions to minimize the risk of negative outcomes.
But in the US and some other countries there is a parallel system of drug regulation that does not do this. Companies are able to sell drugs without studying their pharmacology, and without providing evidence of safety or effectiveness. Many studies have shown that these alternate drugs are commonly adulterated with unlisted ingredients, often don’t have the key ingredient on the label and have substitutions, are contaminated with sometimes toxic substances, and have little quality control in terms of dosing. Often the active ingredients, if any, are not even known, and we have little knowledge about organ toxicity or drug-drug interactions.
This alternate drug regulatory scheme refers to supplements. In the US, since the 1994 DSHEA law, supplements can include herbal drugs and can make health claims, with the only restriction that they cannot name a disease specifically. So the industry has become expert at making sort-of claims, like boosting the immune system or supporting healthy brain function. There is no evidence that granting expanded over-the-counter access to a wide list of herbal drugs has been a positive things for American’s health. Rather, this is a multi-billion dollar predatory industry that, if anything, has worsened overall public health (because they contain risk with no proven benefit). Real nutritional supplements, like vitamins, minerals, and other micronutrients, were already on the market. What DSHEA added was open access to these dirty and poorly controlled drugs.
Continue Reading »
Sep
11
2020
 By now most people have heard that AstraZeneca, a UK pharmaceutical, working with Oxford University, are one of the major companies developing a vaccine for SARS-CoV-2, and also that they have had to pause their Phase 3 clinical trial because a subject came down with an inflammatory disorder. Let’s put this into some important context.
By now most people have heard that AstraZeneca, a UK pharmaceutical, working with Oxford University, are one of the major companies developing a vaccine for SARS-CoV-2, and also that they have had to pause their Phase 3 clinical trial because a subject came down with an inflammatory disorder. Let’s put this into some important context.
The basic facts are that the AstraZeneca vaccine did very well in Phase 1 and 2 preliminary trials. These are smaller trials mostly about safety, with the Phase 2 trial including some preliminary (usually open label) efficacy data. These trials are basically used to determine if it is safe and worth it to proceed to a huge Phase 3 trial. The Phase 3 trial includes 30,000 subjects. When you increase the number of subjects by orders of magnitude then you are likely to pick up increasingly rare side effects. That is one of the main points of this staged approach to research. Then, of course, a drug or vaccine might be marketed to millions of people, and still more rare side effects will crop up. There is simply no way to avoid this – it’s math. That is why so-called Phase 4 trials follow reported side effects after market.
But also, when you are studying 30,000 subjects all the things that normally happen to people will happen at the background frequency. Some of them will get sick during the trial by chance alone, having nothing to do with the study drug or vaccine. So every potential adverse effect is tracked, determined if it is biologically likely that it is related to the experimental treatment, and then statistically analyzed to see if it is above the background rate.
In this case one subject developed transverse myelitis, which is inflammation in one segment of the spinal cord. This will cause weakness and numbness at that level and below, therefore usually affecting the legs. The background incidence of transverse myelitis is about 1.3-4.6 cases per million people per year (this does not include people known to have an autoimmune disease like MS that causes transverse myelitis). If we take 4 cases per million per year, that translates to 0.033 cases per 33,000 subjects over three months. That is the probability that one of the subjects in that trial would have randomly developed transverse myelitis. That may seem really unlikely, but actually you have to consider the probability of a subject developing any disease, not just transverse myelitis. When you add it all up it’s actually pretty likely that one or several people in the trial would randomly develop a disease not related to the vaccine. In fact, this is the second person in this particular trial to develop a serious potential adverse event resulting in a pause of the trial.
Continue Reading »
 Would you be willing to pay $35 for a sticker you put on the back of your phone? What if it had “magical” properties that protect you from something that is not harmful in the first place? That is the idea (it seems to me) behind the SmartDot product, made in the UK. On Amazon they claim: “smartDOT Radiation Protection is a low powered magnet programmed with an intelligent combination of natural harmonizing frequencies which reduces harmful EMF radiation emitted by your wireless devices and alleviate symptoms of electro-stress.”
Would you be willing to pay $35 for a sticker you put on the back of your phone? What if it had “magical” properties that protect you from something that is not harmful in the first place? That is the idea (it seems to me) behind the SmartDot product, made in the UK. On Amazon they claim: “smartDOT Radiation Protection is a low powered magnet programmed with an intelligent combination of natural harmonizing frequencies which reduces harmful EMF radiation emitted by your wireless devices and alleviate symptoms of electro-stress.”
 As the first crop of SARS-CoV-2 vaccines are being rolled out, we can start to see the light at the end of this pandemic tunnel. Still, in the US alone there will probably be another 200,000 deaths before we reach herd immunity. Further, as fast as this vaccine development and deployment was, it’s not clear how much faster it will bring an end to the pandemic. We will likely be 18 months into the pandemic before we have significant vaccine uptake, and that may not be far off from how long the pandemic was going to last in the first place. There is no doubt is will save lives and hasten the end, but still – only after we lost about half a million people to the virus.
As the first crop of SARS-CoV-2 vaccines are being rolled out, we can start to see the light at the end of this pandemic tunnel. Still, in the US alone there will probably be another 200,000 deaths before we reach herd immunity. Further, as fast as this vaccine development and deployment was, it’s not clear how much faster it will bring an end to the pandemic. We will likely be 18 months into the pandemic before we have significant vaccine uptake, and that may not be far off from how long the pandemic was going to last in the first place. There is no doubt is will save lives and hasten the end, but still – only after we lost about half a million people to the virus. This week should see the first people in the US to actually receive an approved (at least EUA) vaccine to prevent SARS-CoV-2. There are three vaccines currently ready to go in the West, the Pfizer vaccine which received it’s EUA in the US on Friday and was already approved in the UK, Moderna which should get approval this week, and the Astra Zeneca vaccine which should not be too far behind. The (arguably) biggest challenge has been met – a massive scientific effort to develop vaccines in record time. This has been a collaboration between government and industry, and shows what we can accomplish with sufficient motivation (which translates into both money and easing red tape).
This week should see the first people in the US to actually receive an approved (at least EUA) vaccine to prevent SARS-CoV-2. There are three vaccines currently ready to go in the West, the Pfizer vaccine which received it’s EUA in the US on Friday and was already approved in the UK, Moderna which should get approval this week, and the Astra Zeneca vaccine which should not be too far behind. The (arguably) biggest challenge has been met – a massive scientific effort to develop vaccines in record time. This has been a collaboration between government and industry, and shows what we can accomplish with sufficient motivation (which translates into both money and easing red tape). I have discussed often before how advances in artificial intelligence (AI) are already transforming our world, but are likely to do so much more in the future (even near term). I am interested in one particular application that I think does not get enough attention – using AI to support clinical decision-making. So I was happy to read that one such project will share in
I have discussed often before how advances in artificial intelligence (AI) are already transforming our world, but are likely to do so much more in the future (even near term). I am interested in one particular application that I think does not get enough attention – using AI to support clinical decision-making. So I was happy to read that one such project will share in  Two weeks ago
Two weeks ago  Over the course of the pandemic the death rate in people diagnosed with COVID-19 (the case-fatality rate) has declined. Unpacking all the reasons this may be the case can help us better understand and fight this disease. A few recent studies shed some light on this question. While there might be some encouraging news here, it highlights that this is still a “novel” virus and we have a lot to learn about the illness it causes.
Over the course of the pandemic the death rate in people diagnosed with COVID-19 (the case-fatality rate) has declined. Unpacking all the reasons this may be the case can help us better understand and fight this disease. A few recent studies shed some light on this question. While there might be some encouraging news here, it highlights that this is still a “novel” virus and we have a lot to learn about the illness it causes. How many people have died in the US so far from the COVID-19 pandemic? It depends on how you count the numbers. The official count of
How many people have died in the US so far from the COVID-19 pandemic? It depends on how you count the numbers. The official count of  One question weighing on the minds of many people today is – when will this all end? And by “this” (well, one of the “thises”) I mean the pandemic. Experts have been saying all along that we need to buckle up and get read for a long ride on the pandemic express. This is a marathon, and we need to be psychologically prepared for what we are doing now being the new normal for a long time. The big question is – what will it take to end the pandemic?
One question weighing on the minds of many people today is – when will this all end? And by “this” (well, one of the “thises”) I mean the pandemic. Experts have been saying all along that we need to buckle up and get read for a long ride on the pandemic express. This is a marathon, and we need to be psychologically prepared for what we are doing now being the new normal for a long time. The big question is – what will it take to end the pandemic? Drugs are regulated in most countries for a reason. They can have powerful effects on the body, can be particularly risky or are incompatible with certain diseases, and can interact with other drugs. Dosages also need to be determined and monitored. So most countries have concluded that prescriptions of powerful drugs should be monitored by physicians who have expertise in their effects and interactions. Some drugs are deemed safe enough that they can be taken over the counter without a prescription, usually in restricted doses, but most require a prescription. Further, before going on the market drug manufacturers have to thoroughly study a drug’s pharmacological activity and determine that it is safe and effective for the conditions claimed. There is a balance here of risk vs benefit – making useful drugs available while taking precautions to minimize the risk of negative outcomes.
Drugs are regulated in most countries for a reason. They can have powerful effects on the body, can be particularly risky or are incompatible with certain diseases, and can interact with other drugs. Dosages also need to be determined and monitored. So most countries have concluded that prescriptions of powerful drugs should be monitored by physicians who have expertise in their effects and interactions. Some drugs are deemed safe enough that they can be taken over the counter without a prescription, usually in restricted doses, but most require a prescription. Further, before going on the market drug manufacturers have to thoroughly study a drug’s pharmacological activity and determine that it is safe and effective for the conditions claimed. There is a balance here of risk vs benefit – making useful drugs available while taking precautions to minimize the risk of negative outcomes. By now most people have heard that AstraZeneca, a UK pharmaceutical, working with Oxford University, are one of the major companies developing a vaccine for SARS-CoV-2, and also that they have had to pause their Phase 3 clinical trial because a subject came down with an inflammatory disorder. Let’s put this into some important context.
By now most people have heard that AstraZeneca, a UK pharmaceutical, working with Oxford University, are one of the major companies developing a vaccine for SARS-CoV-2, and also that they have had to pause their Phase 3 clinical trial because a subject came down with an inflammatory disorder. Let’s put this into some important context.




