Sep 28 2021
Light Beads Microscopy To Image Brain Activity
 The current estimate is that the average human brain contains 86 billion neurons. These neurons connect to each other in a complex network, involving 100 trillion connections. The job of neuroscientists is to map all these connections and to see how they work – no small task. There are multiple ways to approach this task.
The current estimate is that the average human brain contains 86 billion neurons. These neurons connect to each other in a complex network, involving 100 trillion connections. The job of neuroscientists is to map all these connections and to see how they work – no small task. There are multiple ways to approach this task.
At first neuroscientists just looked at the brain and described its macroscopic (or “gross”) anatomical structures. We can see there are different lobes of the brain and major connecting cables. You can also slice up the brain and see its internal structure. When the microscope was developed we could then look at the microscopic structure of the brain, and by using different staining techniques we could visualize the branching structure of axons and dendrites (the parts of neurons that connect to other neurons), we could see that there were different kinds of neurons, various layers in the cortex, and lots of pathways and nodes.
But even when we had a detailed map of the neuroanatomy of the brain down to the microscopic level, we still needed to know how it all functioned. We needed to see neurons in action. (And further, there are lots of non-neuronal cells in the brain such as astrocytes that also affect brain function.) At first we were able to infer what different parts of the brain did by examining people who had damage to one part of the brain. Damage to the left temporal lobe in most people causes language deficits, so this part of the brain must be involved in language processing. We could also do research on animals for all but the highest brain functions.
As new techniques were added, our ability to map brain function grew. The brain is essentially an electrical organ, so we could measure that electrical (and later magnetic) activity to see the activity in different parts of the brain in real time. Modern computers allow for very detailed analysis of these electrical signals. We could also electrically stimulate the brain to see what happens – the opposite approach of seeing what damage does. Another approach to imaging brain function was looking at metabolism. Neurons are hungry because doing what they do uses up a lot of energy, so we could look at markers of metabolism to see which clumps of brain cells were active. PET scans and functional MRI scans use this technique.
With each new technique, our knowledge of the brains connections and their functions (the connectome) grew. But again – with 86 billion neurons and 100 trillion connections, it’s clear we are still far from a complete map of the connectome. Further, brain organization and function is extremely complex, challenging even our basic concepts of how it works. For example, can we understand the brain as a series of networks of neurons, or as a combination of modules (nodes) of neurons with a specific function? It seems that the answer is probably both – there are networks of modules, and those modules can serve different functions when participating in different networks. We therefore cannot even answer the question of – what does this bit of the brain do. The real answer is, it depends, on what networks this particular bit of the brain is currently participating in.
If we want to fully understand brain function one method would be to detect in real time the full activity of all 86 billion neurons. Another method would be to model the activity of 86 billion neurons in a supercomputer, and these two methods are complementary. But we are nowhere close to being able to image the brain’s activity at this ultimate level of detail. Although advancing all the time, current technology has low resolution compared to the complexity of the brain. Some recent progress shows roughly where we are.
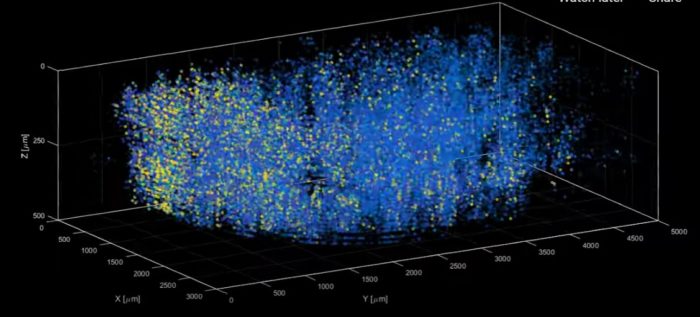
A technique known as light beads microscopy has been able (in a mouse) to image the real-time simultaneous activity of one million neurons. That is quite a feat, although still almost four orders of magnitude less than the entire human brain. The new paper is titled: High-speed, cortex-wide volumetric recording of neuroactivity at cellular resolution using light beads microscopy.
This builds on a technique of using lasers to activate fluorescent tags in neurons. A laser is fired for a few nanoseconds at a neuron with a fluorescent tag, causing the neuron to light up briefly and in such a way that its current state of activity can be inferred. This technique works in tissue that scatters light, and is therefore non-transparent (like the brain). While powerful, the technique has a significant trade-off in which higher resolution and volume comes at a significant loss of speed. This is there the new advance comes in.
The researchers were able to tweak this technique to obtain essentially maximal speed:
The technique involves breaking one strong pulse into 30 smaller sub pulses – each at a different strength – that dive into 30 different depths of scattering mouse brain but induce the same amount of fluorescence at each depth. This is accomplished with a cavity of mirrors that staggers the firing of each pulse in time and ensures that they can all reach their target depths via a single microscope focusing lens.
With this technique the limiting factor on how fast they can record neuronal activity is the time it takes for the fluorescent tag itself to light up. This is therefore essentially as fast as this technique can get. The researchers were able to demonstrate the technique by visualizing one million neurons simultaneously across the entire mouse brain. There is further good news in that this technique uses the same equipment as two-photon microscopy already in use, to it can be rapidly adopted by neuroscience labs around the world.
We still have a long way to go before we can measure and model the simultaneous activity of an entire human brain. But a million neurons is still a powerful tool for understanding brain function. Hopefully we’ll start to see some results from this technique soon. It’s also possible that further incremental advances will make it even more powerful going forward.






